��Ŀ����
����Ŀ����������10mL0.1mol/L��HR��Һ������0.1mol/L��NH3��H2O��Һ, ������ҺpH�������Ա仯��ͼ�����з�������ȷ����
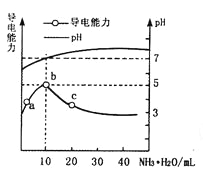
A��a��b�㵼��������ǿ��˵��HRΪ����
B��b����ҺpH=7��˵��NH4Rû��ˮ��
C��c����Һ����c(NH4+)>c(R-)��c(OH-)>c(H+)
D��b��c�������Һ����c(H+)��c(OH-)=Kw=1.0��10-14
���𰸡�B
��������
��������������£���10 mL o��1 mol/L��HR��Һ����ε���0.l mol/L��NH3��H2O��Һ��A��a~b�㵼��������ǿ��������ӦΪHR+NH3��H2O=NH4R+ H2O����Һ����������ǿ��˵����Һ�е��Ũ������ԭ��Һ�е��Ũ���٣�����λ�������Һ�������ƶ��������٣���HRΪ������ʣ���A����ȷ��B��b�㵼��������ǿ����b��λ����ǡ����ȫ��Ӧ����Һ����ΪNH4R ��NH4+��R����Ӧ�ļ���Ϊ������ʣ������߶���ˮ�⣬pH =7��˵������ˮ��̶���ͬ����B�����C��c����ҺΪNH4R��NH3��H2O1:1�Ļ��Һ������ҺpH>7���ʴ���c(OH-)>c(H+)����ΪNH3��H2O�ĵ���̶ȴ���NH4+��ˮ��̶ȣ���c( NH4+)>c( R-)��C����ȷ��D����������ϵ�о�Ϊˮ��Һ��ϵ���ʳ����£�����������c(H+)��c(OH-)= Kw =l. 0��l0-14����D����ȷ������ѡB��

 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д�����Ŀ�����ڷ��ֵ�һ����Ȼ��ʮ������������Al��Cu.Fe���ֽ���Ԫ����ɣ��ش��������⣺
��1����ͭԪ��λ�����ڱ���_____����Cu2+���ӵļ۲�����ʾʽΪ____��
���̡�����������Ԫ�ص����������±���
������/KJ/mol | I1 | I2 | I 3 | I4 |
Mn | 717.3 | 1509.0 | 3248 | 4940 |
Fe | 762.5 | 1561.9 | 2957 | 5290 |
Co | 760.4 | 1648 | 3232 | 4950 |
��Ԫ�صĵ������������Ե�����Ԫ�غ���Ԫ�أ���ԭ����____��
��ʵ���ҿ��ó�Ѫ��K3[Fe(CN)6]����Fe2+���ӣ��ڳ�Ѫ������Ԫ�صĻ��ϼ�Ϊ____���������ӵ���λ��Ϊ______��
��2�����÷�Ӧ��X+C2H2+NH3��Cu2C2+NH4Cl(δ��ƽ)�ɼ�����Ȳ��
�ٻ�����X�����ṹ��ͼ���ݴ˿�֪X�Ļ�ѧʽΪ_______��
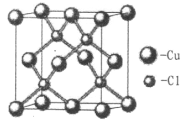
����Ȳ�����ЦҼ���м���Ŀ֮��Ϊ______��̼ԭ�ӵ��ӻ���ʽΪ_______��NH4+�ռ乹��Ϊ______����������������
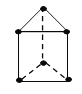
��3�����������ֻ�����a��AlCl3 b��NaCl c��Al2O3�е��ɸߵ���������_______�����ţ�����ԭ����____________��
��Al������ԭ�Ӳ�ȡ�����������ܶѻ����侧���߳�Ϊ0.405nm����ʽ��ʾAl���ʵ��ܶ�_______g/cm3�����ؼ�����������
����Ŀ�����¶�T1��T2ʱ���ֱ�0.50 mol CH4��1.20 mol NO2�������Ϊ1L���ܱ������У��������·�Ӧ��CH4��g��+2NO2��g��N2��g��+CO2��g��+2H2O��g�������n��CH4����ʱ��仯�������±���
ʱ��/min | 0 | 10 | 20 | 40 | 50 | |
T1 | n��CH4��/mol | 0.50 | 0.35 | 0.25 | 0.10 | 0.10 |
T2 | n��CH4��/mol | 0.50 | 0.30 | 0.18 | �� | 0.15 |
����˵����ȷ���ǣ�������
A. T2ʱ��NO2��ƽ��ת����Ϊ70.0%
B. �÷�Ӧ����H��0��T1��T2
C. ���������������䣬T1ʱ��ƽ����ϵ���ٳ���0.30molCH4��0.80molH2O��g����ƽ��������Ӧ�����ƶ�
D. ���������������䣬T1ʱ��ƽ����ϵ���ٳ���0.50molCH4��1.20molNO2����ԭƽ����ȣ�����ƽ��ʱN2��Ũ���������������С