��Ŀ����
����Ŀ�������ֶ�����Ԫ�أ����ǵĽṹ�����ʵ���Ϣ���±�������

����ݱ�����Ϣ��д��
��1��Aԭ�ӵĺ�������Ų�ʽΪ________________��
��2��BԪ�������ڱ��е�λ����____�����Ӱ뾶��B_____A(��������������С����)��
��3��Cԭ�ӵĵ����Ų�ͼ��________����ԭ�Ӻ�����________��δ�ɶԵ��ӣ�������ߵĵ���Ϊ________����ϵĵ��ӣ�������________�Ρ�
��4��Dԭ�ӵ���Χ�����Ų�ʽΪ____________��D���Ľṹʾ��ͼ��____________��
��5��B������������Ӧ��ˮ������A������������Ӧ��ˮ���ﷴӦ�Ļ�ѧ����ʽΪ________��B������������Ӧ��ˮ������D���⻯���ˮ��Һ��Ӧ�Ļ�ѧ����ʽΪ__________________��
���𰸡���1��1s22s22p63s1
��2����3���ڵ���A�� С��
��3��![]() 3 p �Ĵ�
3 p �Ĵ�
��4�� 3s23p5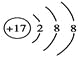
��5��NaOH��Al(OH)3===NaAlO2��2H2O 3HCl��Al(OH)3===AlCl3��3H2O
������������������Ƕ�������(��ϡ��������)ԭ�Ӱ뾶����Ԫ�أ���Ԫ�ص�ij�ֺϽ���ԭ�ӷ�Ӧ�ѵĵ��ȼ���A��Na��B��Aͬ���ڣ�������������ˮ��������ԣ�B��Al��Ԫ�ص���̬�⻯�K������ˮ���������������C��N���Ǻ�ˮ�г��⡢��Ԫ���⺬������Ԫ�أ��䵥�ʻ���Ҳ����ˮ���������г��õ�����ɱ������D��Cl��
��1�� Naԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s1��
��2�� AlԪ�������ڱ��е�λ���ǵ�3���ڵ���A�壻���Ӱ뾶��BС��A��
��3��Nԭ�ӵĵ����Ų�ͼ��![]() ����ԭ�Ӻ�����3��δ�ɶԵ��ӣ�������ߵĵ���Ϊp����ϵĵ��ӣ������ʷĴ��Ρ�
����ԭ�Ӻ�����3��δ�ɶԵ��ӣ�������ߵĵ���Ϊp����ϵĵ��ӣ������ʷĴ��Ρ�
��4�� Clԭ�ӵ���Χ�����Ų�ʽΪ3s23p5��D���Ľṹʾ��ͼ��![]() ��
��
��5����������������������Ӧ�Ļ�ѧ����ʽΪ)NaOH��Al(OH)3===NaAlO2��2H2O�������������ᷴӦ�Ļ�ѧ����ʽΪ3HCl��Al(OH)3===AlCl3��3H2O��

����Ŀ���״�����Ҫ�Ļ���ԭ�ϣ��ֿɳ�Ϊȼ�ϡ����úϳ�������Ҫ�ɷ�ΪCO��CO2��H2���ڴ����������ºϳɼ״�������������Ӧ���£�
��CO(g)+2H2(g)![]() CH3OH(g) ��H1
CH3OH(g) ��H1
��CO2(g)+3H2(g)![]() CH3OH��g��+H2O(g) ��H2
CH3OH��g��+H2O(g) ��H2
��CO2(g)+H2(g)![]() CO(g)+H2O(g) ��H3
CO(g)+H2O(g) ��H3
��1����֪��Ӧ���е���صĻ�ѧ�������������£�
��ѧ�� | H��H | C��O | C��O | H��O | C��H |
E/(kJ��mol-1) | 436 | 343 | 1076 | 465 | 413 |
�ɴ˼����H1=__________kJ��mol-1����֪��H2=-58kJ��mol-1�����H3=_________kJ��mol-1
��2����Ӧ�ٵĻ�ѧƽ�ⳣ��K�ı���ʽΪ_______________���۵Ļ�ѧƽ�ⳣ��K�ı���ʽΪ_____________��