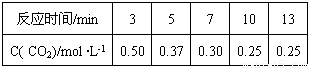
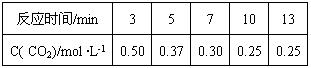
��Ŀ����
Ԫ��X��Y��Z��Wλ��Ԫ�����ڱ���ǰ�����ڣ�ԭ���������������������Ϣ���£�
|
Ԫ�� |
�� �� �� Ϣ |
|
X |
��̬ԭ�Ӻ����������ܼ���ÿ���ܼ��ĵ���������� |
|
Y |
Y��Z����ͬһ���ڣ���ԭ�Ӻ���δ�ɶԵ������ȵ��Ӳ�����1 |
|
Z |
����W�γ����ֻ������ˮ��Һ�������� |
|
W |
�ǵؿ��������ٷֺ����ڶ��Ľ���Ԫ�� |
��ش�
��1��W�Ļ�̬ԭ�Ӻ�������Ų�ʽ��__________��Y���ʷ����к� ���м�,�ԱȽ�Y��ͬ�������ڵ�����Ԫ�صĵ�һ�����ܴ�С��ϵ_____>_____>_____(��Ԫ�ط���)
��2��X��Z�γɵ���Ļ�������һ���������ܼ��������� ������ԡ� �����Ǽ��ԡ��� �����ɵ� ������ԡ������Ǽ��ԡ�������
��3��һ��������Z��Y���γ�YZ3��YZ3��ˮ��Ӧ������һ�������һ�������д���÷�Ӧ�ķ���ʽ______________________________
��4��X���γɵ�һ�־�����и�Ӳ�ȡ����۵����ԣ�����______������ӡ��� ��ԭ�ӡ������ӡ������壬�þ���ṹ��Z��Z��Z����Ϊ_______��
��1��1s22s22p63s23p63d64s2����[Ar]3d64s2����2�֣� 2��2�֣� N>O>C ��2�֣�
��2�����Լ� �Ǽ��� ��2�֣� ��3��NCl3 + 4H2O = NH3 .H2O + 3HClO��3�֣�
(4)ԭ�ӣ�1�֣�109��28�䣨1�֣�
����������

 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д�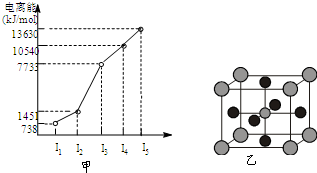 ��X��Y��Z��W����Ԫ�أ�ԭ���������μ�С����֪X�ǵ������ڵ�����Ԫ�أ��䲿�ֵ�������ͼ����ʾ��X��YԪ�ؾ�����ͬ����������ϼۣ�Z ԭ��p�������3�����ӣ�Wԭ�Ӽ۵����Ų�ʽΪnsnnpn��
��X��Y��Z��W����Ԫ�أ�ԭ���������μ�С����֪X�ǵ������ڵ�����Ԫ�أ��䲿�ֵ�������ͼ����ʾ��X��YԪ�ؾ�����ͬ����������ϼۣ�Z ԭ��p�������3�����ӣ�Wԭ�Ӽ۵����Ų�ʽΪnsnnpn�� CH3OH(g) +H2O(g)
CH3OH(g) +H2O(g)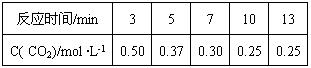
 CH3OH(g)
+H2O(g)
CH3OH(g)
+H2O(g)