��Ŀ����
��8�֣���һ��ɫ��Һ�����п��ܺ���Fe3+��Al3+��Fe2+��Mg2+��Cu2+��NH4+��K+��CO32����SO42�������ӵļ��֣�Ϊ������ɷ֣�ȡ����Һ�ֱ�������ĸ�ʵ�飬��������й���������ͼ��ʾ��


 ��1����ԭ��Һ��һ�������ڵ�������
��
��1����ԭ��Һ��һ�������ڵ�������
��
��2���ڢ۸�ʵ���У���ȡԭ��Һ�����Ϊ100 mL���μӵ�NaOH��Һ�����ʵ���Ũ��Ϊ0.5 mol��L��1 �����ɰ�ɫ�������������NaOH��������ͼ��ʾ�����ϵ�������Һ�����������ӵ����ʵ���Ũ��Ϊ ��

���𰸡�
��1��Fe3+��Fe2+��Cu2+��NH4+��K+��CO32����4�֣���1�ֿ�1�֣���дһ�ֿ�1�֣���д���ֲ��÷֣���
��2��c(Al3+) = 0.5 mol��L��1 ��2�֣� c(Mg2+) = 0.2 mol��L��1 ��2�֣�
��������

��ϰ��ϵ�д�
�����Ŀ

 ��ʾ�����ϵ���ݴ˿�֪��
��ʾ�����ϵ���ݴ˿�֪��


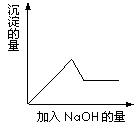 �ڢ۸�ʵ���У����ɰ�ɫ�������������NaOH��������ͼ
�ڢ۸�ʵ���У����ɰ�ɫ�������������NaOH��������ͼ
 ��ʾ�����ϵ���ݴ˿�֪��
��ʾ�����ϵ���ݴ˿�֪��