��Ŀ����
��һ��ɫ��Һ�����п��ܺ���Fe3+��Al3+��Fe2+��Mg2+��Cu2+��NH4+��K+��CO32-��SO42-�����ӵļ��֣�Ϊ������ɷ֣�ȡ����Һ�ֱ�������ĸ�ʵ�飬��������й���������ͼ��ʾ��

�ڢ۸�ʵ���У����ɰ�ɫ�������������NaOH��������ͼ��ʾ�����ϵ���ݴ˿�֪��

����ԭ��Һ��һ�������ڵ�������_____________________��
��Ϊ�������Һ��һ�����ڵ����ӵ�Ҫ��һ����ܽ����ֳ�������Ϊ��д��ѧʽ��___________��_________��
��д���ڢ۸�ʵ���з�����Ӧ�����ӷ���ʽ ��
��Ϊ�������Һ��һ�����ڵ����ӵ�Ҫ��һ����ܽ����ֳ�������Ϊ��д��ѧʽ��___________��_________��
��д���ڢ۸�ʵ���з�����Ӧ�����ӷ���ʽ ��
��1��Fe3+��Fe2+��Cu2+��NH4+��CO32-��
��2��KAl(SO4)2����KAl(SO4)2��12H2O����MgSO4
��3��Mg2++2OH-=Mg(OH)2��
Al3++3OH-=Al(OH)3��
Al(OH)3+OH-=AlO2-+2H2O
��2��KAl(SO4)2����KAl(SO4)2��12H2O����MgSO4
��3��Mg2++2OH-=Mg(OH)2��
Al3++3OH-=Al(OH)3��
Al(OH)3+OH-=AlO2-+2H2O

��ϰ��ϵ�д�
�����Ŀ

 ��ʾ�����ϵ���ݴ˿�֪��
��ʾ�����ϵ���ݴ˿�֪��
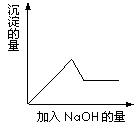 �ڢ۸�ʵ���У����ɰ�ɫ�������������NaOH��������ͼ
�ڢ۸�ʵ���У����ɰ�ɫ�������������NaOH��������ͼ
 ��ʾ�����ϵ���ݴ˿�֪��
��ʾ�����ϵ���ݴ˿�֪��