��Ŀ����
��һ��ɫ��Һ�����п��ܺ���Fe3+��Al3+��Fe2+��Mg2+��Cu2+��NH4+��K+��CO32-��SO42-�������еļ��֣�Ϊ������ɷ֣�ȡ����Һ�ֱ�������ĸ�ʵ�飬��������й���������ͼ��ʾ��

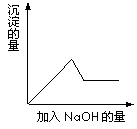 �ڢ۸�ʵ���У����ɰ�ɫ�������������NaOH��������ͼ
�ڢ۸�ʵ���У����ɰ�ɫ�������������NaOH��������ͼ
��ʾ�����ϵ���ݴ˿�֪��
����ԭ��Һ��һ�������ڵ�������____________________��
��Ϊ�������Һ��һ�����ڵ����ӵ�Ҫ��һ����ܽ���
�ֳ������ʣ��仯ѧʽ�ֱ�Ϊ___________��_________��
��д���ڢ۸�ʵ���п��ܷ��������з�Ӧ�����ӷ���ʽ__________��
��1��Fe3+��Fe2+��Cu2+��NH4+��CO32-��
��2��KAl(SO4)2����KAl(SO4)2?12H2O�� ���� MgSO4
��3��Mg2++2OH-=Mg(OH)2�� Al3++3OH-=Al(OH)3�� Al(OH)3+OH-=AlO2-+2H2O
����д����������һ����Al3++4OH-=AlO2-+2H2O����۷֣�


 ��ʾ�����ϵ���ݴ˿�֪��
��ʾ�����ϵ���ݴ˿�֪��


 ��ʾ�����ϵ���ݴ˿�֪��
��ʾ�����ϵ���ݴ˿�֪��