��Ŀ����
����Ŀ������β���к����ϰ��ֻ������Ⱦ������NOΪ������ش�������NO��ص����⣺
��1����������������ʱȼ��ȼ�ջ�ʹ����ת��ΪNO��
��֪:N2(g)+O2(g)=2NO(g) ��H=+akJ��mol-1��NO�е���������Ϊb kJ��mol-1��O=O����Ϊ c kJ��mol-1��
����N_N�ļ���Ϊ____________kJ��mol-1���ú�a��b��c�Ĵ���ʽ��ʾ����
��2��NO��CO��һ���¶Ⱥʹ����������¿ɷ�����Ӧ��
2NO(g)+2CO(g)![]() N2(g)+2CO2(g) ��H=-538 kJ��mol-1��
N2(g)+2CO2(g) ��H=-538 kJ��mol-1��
һ���¶��£������Ϊ2L���ܱ������г���һ������NO��CO������������Ӧ����Ӧ�����вⶨ�IJ������ݼ��±���
ʱ��/min | n(NO)/mol | n(CO)/mol | n(N2)/mol | n(CO2)/mol |
0 | 0.100 | 0.200 | 0 | 0 |
4 | 0.020 | |||
6 | 0.050 | |||
8 | 0.025 |
�ٷ�Ӧ��0~4 min�ڵ�ƽ������v(CO)=_____________��
�����������У���˵����Ӧ�Ѵﵽƽ�����____________��
a��NO���������ʺ�CO2������������� b�������ڵ�ѹǿ���ٱ仯
c�����������ܶȲ��ٱ仯 d��N2�İٷֺ������ٱ仯
�ۼ�����¶��µ�ƽ�n����K=____________��
��8minʱ�������������䣬��÷�Ӧ��������ͨ��0.05molNO��0.100CO�����´ﵽƽ��ʱ���������N2�İٷֺ�����____________�����С�����������䡱 ����
��3����ҵ�ϲ��õ��NO�ķ����Ʊ�NH4NO3���乤��ԭ����ͼ��X��Y��ΪPt�缫��Ϊʹ������ȫ��ת��ΪNH4NO3���貹��NH3��
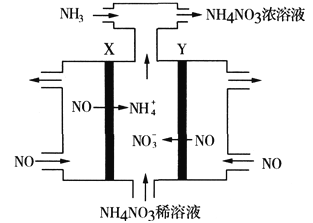
��Y�缫�ϵĵ缫��ӦʽΪ_______________________________��
����X�缫����11.2L���ѻ���ɱ�״����NO������Ӧ��������NH4NO3������Ϊ____________g��
���𰸡� (2b +a-c) 0.005mol��L-1.min-1 b d 2.2 L/mol ���� NO -3e-+ 2H2O == NO3-+ 4H+ 66.7g
����������1������NO��O2�����л�ѧ���ļ��ֱܷ���bkJmol-1��ckJmol-1�Լ���ӦN2��g��+O2��g��=2NO��g����H=+akJmol-1������N2�����л�ѧ���ļ���ΪX�����У�xkJmol-1+ckJmol-1-2b=akJmol-1���ã�X=(a+2b-c)kJmol-1��
��2���ٷ�Ӧ��0~4 min�ڵ�ƽ������v(CO)=2v(N2)=2�� =0.005mol��L-1.min-1��
=0.005mol��L-1.min-1��
��a��NO���������ʺ�CO2���������ʾ�Ϊ����Ӧ���ʣ����ж�ƽ��״̬����a����b������������䣬�����������ʵ�����ȷ������ѹǿ���ٱ仯˵�������ʵ���һ������ƽ��״̬����b��ȷ��c�����������ʼ�ղ��䣬���������ܶ�ʼ�ղ��䣬���ж���ƽ��״̬����c����d��N2�İٷֺ������ٱ仯����һ������ƽ��״̬����d��ȷ����Ϊbd��
�۷�Ӧ���е�6minʱCO2�����ʵ���Ϊ0.05mol����ʱN2�����ʵ���Ϊ0.025mol����8minʱN2�����ʵ�����ͬ����6minʱ��Ӧ�ﵽƽ�⣬ƽ��״̬��NO�����ʵ���Ϊ0.100mol-0.050mol=0.050mol��CO�����ʵ���Ϊ0.200mol-0.050mol=0.150mol�����¶��µ�ƽ�n����K=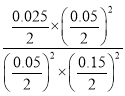 =2.2��
=2.2��
��8minʱ�������������䣬��÷�Ӧ��������ͨ��0.05molNO��0.100CO��ƽ�������ƶ������´ﵽƽ��ʱ���������N2�İٷֺ�����������
��3���ٵ��NO�Ʊ�NH4NO3����ͼ��֪����Y�����ķ�ӦΪNO-3e-+2H2O=NO3-+4H+��
������X����ӦΪ��NO+5e-+6H+=NH4++H2O������Y�����ķ�ӦΪNO-3e-+2H2O=NO3-+4H+����������Ӧ�ɿ�����Ҫʹ��ʧ�����غ㣬����������NO3-�����ʵ�����������������NH4+�����ʵ������ܷ�Ӧ����ʽΪ��8NO+7H2O![]() 3NH4NO3+2HNO3�������䰱��������ȫ����������泥���8NO+7H2O+2NH3
3NH4NO3+2HNO3�������䰱��������ȫ����������泥���8NO+7H2O+2NH3![]() 5NH4NO3����X�缫����11.2L��0.5molNO������Ӧ�����ݵ����غ��֪����������Ӧ��NO�����ʵ���Ϊ0.5mol��
5NH4NO3����X�缫����11.2L��0.5molNO������Ӧ�����ݵ����غ��֪����������Ӧ��NO�����ʵ���Ϊ0.5mol��![]() =
=![]() mol��ʵ�ʲμӷ�Ӧ��NO�����ʵ���Ϊ��0.5+
mol��ʵ�ʲμӷ�Ӧ��NO�����ʵ���Ϊ��0.5+![]() ��mol=
��mol=![]() mol������NH4NO3�����ʵ���Ϊ0.5mol��
mol������NH4NO3�����ʵ���Ϊ0.5mol��![]() ������Ϊ
������Ϊ![]() mol��
mol��![]() ��80g/mol=66.7g��
��80g/mol=66.7g��

 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д� ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�����Ŀ��ij��ҩ������C2H4��ˮ�����Լ�C4H8�ϳ�һ��ҩ��K����ϳ�·��������£�
| |
D����NaHCO3��Һ�����������壬��FeCl3��Һ������ɫ��G�����ԣ�H��I�����ԡ�
(1)��������������ȡ����Ӧ����_____________________��
(2)C�ṹ�й����ŵĵ���ʽΪ_______________��E�Ľṹ��ʽΪ__________________________��
(3)��Ӧ�ߵĻ�ѧ����ʽΪ______________________________________________��
(4)D������NaOH��Һ���ȵĻ�ѧ����ʽΪ_________________________________________________________��
(5)I��������״��������ͬ���칹�干��_________��(�����������칹)��



