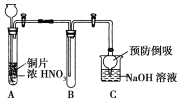
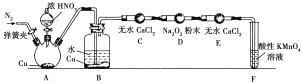
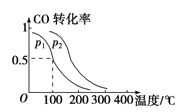
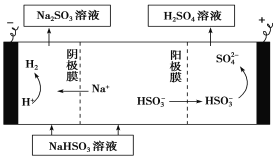
��Ŀ����
����Ŀ���л����У���һЩ����ʽ����ͨʽCnHn����C2H2��C6H6�ȡ�
(1)������Щ�л����˵������ȷ����______(����)��
A�����ܻ�Ϊͬϵ�� |
B���ڿ�����ȼ��ʱ���������Ҳ������� |
C��һ����ʹ�������������Һ��ɫ |
D������������Щ�л�����ȫȼ��ʱ��������ͬ |
(2)д������ʽΪC4H4��Ϊ�������л���Ľṹ��ʽ__________������һ�ȴ����ͬ���칹����________�֡�
(3)д�����������ʽΪC6H6�ҽṹ��ֻ��C��C����C��H�����л���Ľṹ��ʽ��____________________________________________________________��
(4)ij�л������ʽΪC8H8�������ڷ���������֪����ʹ�������������Һ����ˮ��ɫ������л���Ľṹ��ʽΪ________��д���䷢���Ӿ۷�Ӧ�Ļ�ѧ����ʽ��____________________________________��
���𰸡�(1)BD��
(2)HC��C��CH==CH2��3��
(3)��
(4)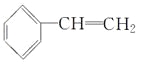 ��
�� ��
��
�������������������1��A��ͬϵ�����Ƚṹ���ƣ�C2H2����Ȳ����C6H6���ڷ�������������ͬϵ��ʴ���B��ͨʽΪCnHn����̼���ߣ��Һ�̼����ȣ����ȼ�չ����л��������Ҳ������̣�����ȷ��C��C6H6����DZ�������ʹ���Ը��������Һ��ɫ���ʴ���D��������ʱ�����ʽΪCH����˺�������ͬ������ȷ��(2)4��C��������������ΪC4H10����C4H4���6���⣬˵������3��̼̼˫����1��̼̼������1��̼̼˫����ͬһ��̼ԭ���ϲ�����������̼̼˫�����������л��ﲻ�ȶ�����˴��л���ΪHC��C��CH=CH2����3�ֲ�ͬ����ԭ�ӣ���һ�ȴ�����3�֣���3��ֻ��C��C����C��H����˵���DZ��͵ģ����������������ʽΪC6H14����˲����������ṹ��ʽֻ����![]() ����4�����ڷ����������б�������ʹ���������Һ����ˮ��ɫ�����в����ͼ�����˽ṹ��ʽΪ��
����4�����ڷ����������б�������ʹ���������Һ����ˮ��ɫ�����в����ͼ�����˽ṹ��ʽΪ�� ��
�� ��
��

 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д�