��Ŀ����
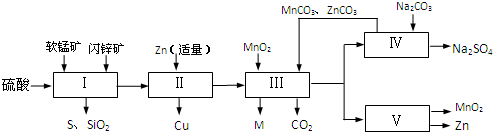
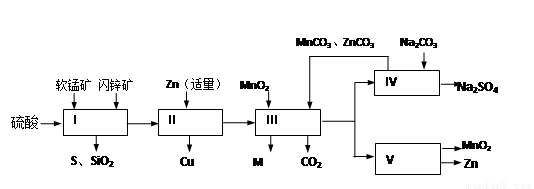
���̿��к�MnO2Լ70%��SiO2Լ20%��Al2O3Լ4%������Ϊˮ�֣���п���к�ZnSԼ80%��FeS��CuS��SiO2��Լ7%������Ϊˮ�֣�������Ա�������ۺ�������������Դ��ͬ��������գ���ȡZn��MnO2��Na2SO4���乤���������£�
| ������ | Fe��OH��3 | Al��OH��3 | Zn��OH��2 |
| ��ʼ����pH | 2.3 | 4.0 | 5.4 |
| ��ȫ����pH | 4.1 | 5.2 | 8.0 |
______��
��2�����е�����Һ��pH��5.2��5.4����ʱ���ɳ���M�ijɷ�Ϊ______��д��ѧʽ�������м���MnO2��������______��
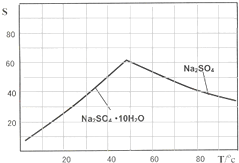
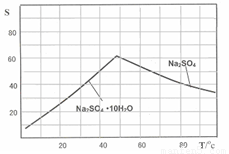
��3����ͼ��Na2SO4��Na2SO4?10H2O���ܽ�����ߣ�g/100gˮ��������еõ�Na2SO4����IJ����ǣ��������MnCO3��ZnCO3�����Һ���½ᾧ��______�����Ҵ�ϴ�Ӻ����Ҵ�ϴ�Ӷ�����ˮϴ��ԭ����______��
��4�������ö��Ե缫����Ƶ�Zn��MnO2���������ĵ缫��ӦʽΪ______��
��5����ɫ��ѧ˼���ڱ������еõ��˳�����֣��ڱ����������п�ѭ��ʹ�õ������У�MnO2��ZnCO3��MnCO3��______��______��д��ѧʽ����
�ʴ�Ϊ��MnO2+CuS+2H2SO4=S��+MnSO4+CuSO4+2H2O��
��2��ͼ�����ݷ�����pH��5.2��5.4ʱ�����Ӻ�������ȫ�����������м���MnO2�������������������������������ڵ�����ҺPHȫ��������
�ʴ�Ϊ��Fe��OH��3��Al��OH��3����Fe2+������Fe3+��
��3��ͼ�������֪�¶ȸ�ʱ���������ƣ��¶ȵ�ʱ���������ƽᾧˮ���ᄃ�壬������Ҫ���ȹ��ˣ�ϴ�Ӿ���ʱ���Ҵ�ϴ�ӱ����γɽᾧˮ���
�ʴ�Ϊ�����ȹ��ˣ���ֹ�γ� Na2SO4?10H2O��
��4������ͼ�еõ�����Ϊ�������̺�п�������õ�п��п�����������õ���������������������������������ʧ�������ɣ��缫��ӦΪ��Mn2+-2e-+2H2O=MnO2+4H+��
�ʴ�Ϊ��Mn2+-2e-+2H2O=MnO2+4H+��
��5����������ͼ�����ʵķ�Ӧ�����ʵ����ɷ�����֪п�������ǿ���ѭ��ʹ�õ��Լ����ʴ�Ϊ��Zn��H2SO4��
��������1����������ͼ�������ɳ�����MnO2��CuS�����ᷴӦ����MnSO4��CuSO4��H2O��������֪����������ԭ��Ӧ��
��2������ͼ�����ݷ�����pH��5.2��5.4ʱ�����Ӻ�������ȫ�����������м���MnO2���������������������������ӣ�
��3������ͼ����ܽ�����¶ȱ仯�����жϣ��õ������Ƶ�����������ע���ܽ�ȵ�Ӱ�죻
��4������ͼ�еõ�����Ϊ�������̺�п�������õ�п��п�����������õ���������������������������ʧ�������ɣ�
��5��������ͼ����Ϊԭ�ϲμӷ�Ӧ��������������ɵ������ǿ���ѭ�����õ����ʷ����жϣ�
���������⿼���������Ʊ�ʵ��ķ����жϣ������ܽ��Ե�����Ӧ�ã����ԭ����Ӧ���жϣ��缫��Ӧ�IJ����жϺ͵缫��Ӧ��д����Ŀ�ѶȽϴ�

���̿��к�MnO2Լ70%��SiO2Լ20%��Al2O3Լ4%������Ϊˮ�֣���п���к�ZnSԼ80%��FeS��CuS��SiO2��Լ7%������Ϊˮ�֡�������Ա�������ۺ�������������Դ��ͬ��������գ���ȡZn��MnO2��Na2SO4���乤���������£�
��1��I����Һ�к���MnSO4��ZnSO4��CuSO4��Fe2(SO4)3��Al2(SO4)3�ȡ�д��MnO2��CuS�����ᷴӦ�Ļ�ѧ����ʽ�� ��
��2����֪Fe(OH)3��Al(OH)3��Zn(OH)2�������ʿ�ʼ��������ȫ����ʱ��Һ��pH���±���
|
������ |
Fe(OH)3 |
Al(OH)3 |
Zn(OH)2 |
|
��ʼ����pH |
2.3 |
4.0 |
5.6 |
|
��ȫ����pH |
4.1 |
5.2 |
8.0 |
��III�е�����Һ��pH��5.2��5.4����ʱ���ɳ���M�ijɷ�Ϊ ��д��ѧʽ����III�м���MnO2�������� ��
��3��Na2SO4��Na2SO4��10H2O���ܽ�����ߣ�g/100gˮ����ͼ����IV�еõ�Na2SO4����IJ����ǣ��������MnCO3��ZnCO3�����Һ���½ᾧ�� �����Ҵ�ϴ�Ӻ������Ҵ�ϴ�Ӷ�����ˮϴ��ԭ���� ��
��4��V���ö��Ե缫����Ƶ�Zn��MnO2���������ĵ缫��ӦʽΪ ��
��5����ɫ��ѧ˼���ڱ������еõ��˳�����֣��ڱ����������п�ѭ��ʹ�õ���Ҫ�����У�MnO2��ZnCO3��MnCO3�� �� ��д��ѧʽ����
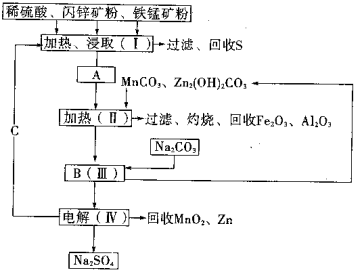 ��2011?����ģ�⣩ij���������̿�MnO2Լ70%������Al2O3������п��ZnSԼ80%������FeS����ͬ����MnO2��Zn���ɵ��ԭ�ϣ������������£�
��2011?����ģ�⣩ij���������̿�MnO2Լ70%������Al2O3������п��ZnSԼ80%������FeS����ͬ����MnO2��Zn���ɵ��ԭ�ϣ������������£�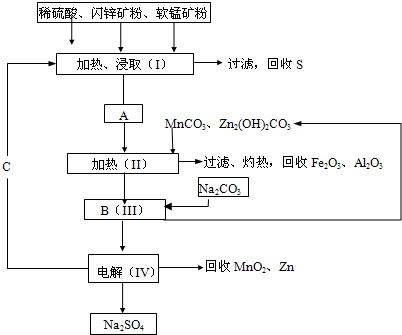
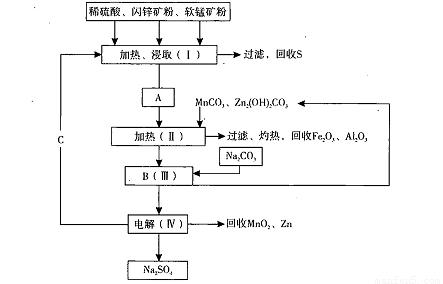
 MnO2+Zn +2H2SO4��
MnO2+Zn +2H2SO4��