��Ŀ����
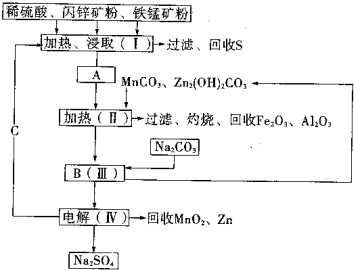 ��2011?����ģ�⣩ij���������̿�MnO2Լ70%������Al2O3������п��ZnSԼ80%������FeS����ͬ����MnO2��Zn���ɵ��ԭ�ϣ������������£�
��2011?����ģ�⣩ij���������̿�MnO2Լ70%������Al2O3������п��ZnSԼ80%������FeS����ͬ����MnO2��Zn���ɵ��ԭ�ϣ������������£���֪����A��MnSO4��ZnSO4��Fe2��SO4��3��Al2��SO4��3�Ļ��Һ��
��IV�еĵ�ⷴӦʽΪ��MnSO4+ZnSO4+2H2O�TMnO2+Zn+2H2SO4
��1��A�����ڻ�ԭ�������
MnSO4
MnSO4
����2�������MnCO3��Zn2��OH��2CO3��������
������Һ��PH��ʹFe3+��Al3+�����ɳ���
������Һ��PH��ʹFe3+��Al3+�����ɳ���
������Ҫ���ȵ�ԭ����
�ٽ�Fe3+��Al3+��ˮ��
�ٽ�Fe3+��Al3+��ˮ��
��C�Ļ�ѧʽ��H2SO4
H2SO4
����3���������г��õ�MnO2��Zn���⣬���ɵõ��ĸ���Ʒ��
Fe2O3��Al2O3��S��Na2SO4
Fe2O3��Al2O3��S��Na2SO4
����4����������������е���ģ�����ʯ�⣬�蹺��Ļ���ԭ����
���������
���������
����5��������MnO2��Zn�ĽǶȼ��㣬���̿����п��������ȴ�Լ��
1.03
1.03
����������1����������ԭ��Ӧ�У����ϼ۽���Ԫ�����ڵIJ����ǻ�ԭ���
��2�����ݿ�ͼ����MnSO4��ZnSO4��Fe2��SO4��3��Al2��SO4��3�Ļ��Һ�м���MnCO3��Zn2��OH��2CO3����Ի������������������
��3������ת�������з����ķ�Ӧ��ȷ���õ��ĸ���Ʒ��
��4����ֿ�ͼ��Ҫ��������ʿ���ȷ��������Ҫ����Ļ���ԭ�ϣ�
��5������IV�еĵ�ⷴӦʽΪ��MnSO4+ZnSO4+2H2O�TMnO2+Zn+2H2SO4����֪MnO2��Zn�����ʵ���֮��Ϊ1��1�����㼴�ɣ�
��2�����ݿ�ͼ����MnSO4��ZnSO4��Fe2��SO4��3��Al2��SO4��3�Ļ��Һ�м���MnCO3��Zn2��OH��2CO3����Ի������������������
��3������ת�������з����ķ�Ӧ��ȷ���õ��ĸ���Ʒ��
��4����ֿ�ͼ��Ҫ��������ʿ���ȷ��������Ҫ����Ļ���ԭ�ϣ�
��5������IV�еĵ�ⷴӦʽΪ��MnSO4+ZnSO4+2H2O�TMnO2+Zn+2H2SO4����֪MnO2��Zn�����ʵ���֮��Ϊ1��1�����㼴�ɣ�
����⣺��1���Ƚ���Ϣ�٣�A�����̿���Ԫ�ػ��ϼ۵ı仯��֪����Ԫ�ػ��ϼ۴�+4�۽���Ϊ+2�ۣ�����A�л�ԭ����ΪMnSO4���ʴ�Ϊ��MnSO4��
��2�����ݿ�ͼ����MnSO4��ZnSO4��Fe2��SO4��3��Al2��SO4��3�Ļ��Һ�м���MnCO3��Zn2��OH��2CO3����Ի���������������������Լ���MnCO3��Zn2��OH��2CO3�����þ��ǵ���pH��ʹFe3+��Al3+������ȫ���ڼ��ȵĻ����£����������ӵ�ˮ������ף������γɳ��������룬����IV�еĵ�ⷴӦʽΪ��MnSO4+ZnSO4+2H2O�TMnO2+Zn+2H2SO4����֪CΪ���ᣬ
�ʴ�Ϊ��������Һ��PH��ʹFe3+��Al3+�����ɳ������ٽ�Fe3+��Al3+��ˮ�⣻H2SO4��
��3����ʵ���Ŀ�ľ����Ʊ�����������п���������Ҫ������������Ԫ�ء���Ԫ�س�ȥ����������ͼ���ѵó��������г��õ�MnO2��Zn���⣬���ɵõ��ĸ���Ʒ���������������������ʴ�Ϊ��Fe2O3��Al2O3��S��Na2SO4��
��4�����ݿ�ͼ��ת�����ĵķ�Ӧ���������������е���ģ�����ʯ�⣬�蹺��Ļ���ԭ�����������ƺ����ᣬ�ʴ�Ϊ����������
��5���ݷ�ӦMnSO4+ZnSO4+2H2O�TMnO2+Zn+2H2SO4��֪MnO2��Zn�����ʵ���֮��Ϊ1��1���ʿ������̿���п��������ֱ�Ϊx��y����
��
=1��1����x��y=1.03���ʴ�Ϊ��1.03��
��2�����ݿ�ͼ����MnSO4��ZnSO4��Fe2��SO4��3��Al2��SO4��3�Ļ��Һ�м���MnCO3��Zn2��OH��2CO3����Ի���������������������Լ���MnCO3��Zn2��OH��2CO3�����þ��ǵ���pH��ʹFe3+��Al3+������ȫ���ڼ��ȵĻ����£����������ӵ�ˮ������ף������γɳ��������룬����IV�еĵ�ⷴӦʽΪ��MnSO4+ZnSO4+2H2O�TMnO2+Zn+2H2SO4����֪CΪ���ᣬ
�ʴ�Ϊ��������Һ��PH��ʹFe3+��Al3+�����ɳ������ٽ�Fe3+��Al3+��ˮ�⣻H2SO4��
��3����ʵ���Ŀ�ľ����Ʊ�����������п���������Ҫ������������Ԫ�ء���Ԫ�س�ȥ����������ͼ���ѵó��������г��õ�MnO2��Zn���⣬���ɵõ��ĸ���Ʒ���������������������ʴ�Ϊ��Fe2O3��Al2O3��S��Na2SO4��
��4�����ݿ�ͼ��ת�����ĵķ�Ӧ���������������е���ģ�����ʯ�⣬�蹺��Ļ���ԭ�����������ƺ����ᣬ�ʴ�Ϊ����������
��5���ݷ�ӦMnSO4+ZnSO4+2H2O�TMnO2+Zn+2H2SO4��֪MnO2��Zn�����ʵ���֮��Ϊ1��1���ʿ������̿���п��������ֱ�Ϊx��y����
| 0.7x |
| 87 |
| 0.8y |
| 97 |
������������һ���йػ����������յ��ۺ�֪ʶ��Ŀ������Ƕȹ㣬�Ѷȴ�

��ϰ��ϵ�д�
�����Ŀ
 ��2011?����ģ�⣩�����ý�屨�������̷����ۡ��°����ס�������֤���ִ����к��л���ù���أ�AFTB������ӽṹʽ��ͼ��ʾ������˵���в���ȷ���ǣ�������
��2011?����ģ�⣩�����ý�屨�������̷����ۡ��°����ס�������֤���ִ����к��л���ù���أ�AFTB������ӽṹʽ��ͼ��ʾ������˵���в���ȷ���ǣ�������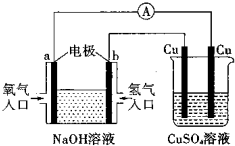 ��2011?����ģ�⣩������ͼ��ʾ��װ�ã��ж�����˵����ȷ���ǣ�������
��2011?����ģ�⣩������ͼ��ʾ��װ�ã��ж�����˵����ȷ���ǣ�������