��Ŀ����
��2008?��ɽһģ��ij���������̿�MnO2Լ70%��Al2O3������п��ZnSԼ80%��FeS����ͬ����MnO2��Zn���ɵ��ԭ�ϣ���
��֪��A��MnSO4��ZnSO4��Fe2��SO4��3��Al2��SO4��3�Ļ��Һ����IV�еĵ�ⷴӦʽΪMnSO4+ZnSO4+2H2O
MnO2+Zn+2H2SO4��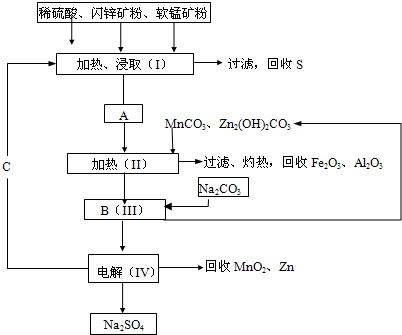
��1��A�����ڻ�ԭ�������
��2��MnCO3��Zn2��OH��2CO3��������
��3���������г��õ�MnO2��Zn���⣬���ɵõ��ĸ���Ʒ��
��4����������������е���ģ�����ʯ�⣬�蹺��Ļ���ԭ����
��5��Ҫ��Na2SO4��Һ�еõ�â����Na2SO4?10H2O��������еIJ���������Ũ����
��6��������MnO2��Zn�ĽǶȼ��㣬���̿����п��������ȴ�Լ��
��֪��A��MnSO4��ZnSO4��Fe2��SO4��3��Al2��SO4��3�Ļ��Һ����IV�еĵ�ⷴӦʽΪMnSO4+ZnSO4+2H2O
| ��� |

��1��A�����ڻ�ԭ�������
MnSO4
MnSO4
����2��MnCO3��Zn2��OH��2CO3��������
������Һ��pH��ʹFe3+��Al3+���ɳ���
������Һ��pH��ʹFe3+��Al3+���ɳ���
��II��Ҫ���ȵ�ԭ�������ٳ������ɣ���ֹ������֣���ʹ�γɽ����������������������Ҳ���ɳ���
���ٳ������ɣ���ֹ������֣���ʹ�γɽ����������������������Ҳ���ɳ���
��C�Ļ�ѧʽ��H2SO4
H2SO4
����3���������г��õ�MnO2��Zn���⣬���ɵõ��ĸ���Ʒ��
����������������
����������������
����4����������������е���ģ�����ʯ�⣬�蹺��Ļ���ԭ����
̼���ơ�����
̼���ơ�����
����5��Ҫ��Na2SO4��Һ�еõ�â����Na2SO4?10H2O��������еIJ���������Ũ����
��ȴ�ᾧ
��ȴ�ᾧ
�����ˡ�ϴ�ӡ�����ȣ���6��������MnO2��Zn�ĽǶȼ��㣬���̿����п��������ȴ�Լ��
1.03
1.03
����������1���Ƚ���Ϣ��A�����̿���Ԫ�ػ��ϼ۵ı仯��֪��A�л�ԭ����ΪMnSO4��
��2���ɹ������̿�֪��A�м���MnCO3��Zn2��OH��2CO3��A�е�Fe3+��Al3+������һϵ�в���ת��Ϊ����������������
Fe3+��Al3+����ʱ�����γɽ��壬������������������������������
��������ȡ���ȡ��Ҫ���ᣬ�ɢڿ�֪CΪ���ᣬѭ�����ã�
��3����������ͼ���ѵó���������������������������
��4������ʯ�⣬���������ʣ�����Ҫ����Ļ���ԭ�ϣ�
��5������Һ�л�ù������ʣ�������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
��6�������̿���п��������ֱ�Ϊx��y�����ݢڿ�֪MnO2��Zn�����ʵ���֮��Ϊ1��1���ݴ��зų�����x��y��ֵ��
��2���ɹ������̿�֪��A�м���MnCO3��Zn2��OH��2CO3��A�е�Fe3+��Al3+������һϵ�в���ת��Ϊ����������������
Fe3+��Al3+����ʱ�����γɽ��壬������������������������������
��������ȡ���ȡ��Ҫ���ᣬ�ɢڿ�֪CΪ���ᣬѭ�����ã�
��3����������ͼ���ѵó���������������������������
��4������ʯ�⣬���������ʣ�����Ҫ����Ļ���ԭ�ϣ�
��5������Һ�л�ù������ʣ�������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
��6�������̿���п��������ֱ�Ϊx��y�����ݢڿ�֪MnO2��Zn�����ʵ���֮��Ϊ1��1���ݴ��зų�����x��y��ֵ��
����⣺��1���Ƚ���Ϣ��A�����̿���Ԫ�ػ��ϼ۵ı仯��֪��MnԪ�ػ��ϼ���+4�۽���Ϊ+2�ۣ�����A�л�ԭ����ΪMnSO4��
�ʴ�Ϊ��MnSO4��
��2���ɹ������̿�֪��MnCO3��Zn2��OH��2CO3�����þ��ǵ���pH��ʹFe3+��Al3+������ȫ��
Fe3+��Al3+���������γɽ��壬���������������������������������м��ȵ�Ŀ���Ǽ��ٳ������ɣ���ֹ������֣���ʹ�γɽ����������������������Ҳ���ɳ�����
��������ȡ���ȡ��Ҫ���ᣬ�ɢڿ�֪CΪ���ᣬѭ�����ã�
�ʴ�Ϊ��������Һ��pH��ʹFe3+�� Al3+���ɳ��������ٳ������ɣ���ֹ������֣���ʹ�γɽ����������������������Ҳ���ɳ�����H2SO4��
��3����������ͼ��֪����������������������������
�ʴ�Ϊ��������������������
��4����������ͼ��֪����Ҫ����̼���ơ����ᣬ���Գ���ʯ�⣬�蹺��Ļ���ԭ����̼���ơ����ᣮ
�ʴ�Ϊ��̼���ơ����ᣮ
��5������Һ�л�ù������ʣ�������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
�ʴ�Ϊ����ȴ�ᾧ��
��6�������̿���п��������ֱ�Ϊxg��yg�����ݢڿ�֪MnO2��Zn�����ʵ���֮��Ϊ1��1����
��
=1��1�����x��y=1.03��
�ʴ�Ϊ��1.03��
�ʴ�Ϊ��MnSO4��
��2���ɹ������̿�֪��MnCO3��Zn2��OH��2CO3�����þ��ǵ���pH��ʹFe3+��Al3+������ȫ��
Fe3+��Al3+���������γɽ��壬���������������������������������м��ȵ�Ŀ���Ǽ��ٳ������ɣ���ֹ������֣���ʹ�γɽ����������������������Ҳ���ɳ�����
��������ȡ���ȡ��Ҫ���ᣬ�ɢڿ�֪CΪ���ᣬѭ�����ã�
�ʴ�Ϊ��������Һ��pH��ʹFe3+�� Al3+���ɳ��������ٳ������ɣ���ֹ������֣���ʹ�γɽ����������������������Ҳ���ɳ�����H2SO4��
��3����������ͼ��֪����������������������������
�ʴ�Ϊ��������������������
��4����������ͼ��֪����Ҫ����̼���ơ����ᣬ���Գ���ʯ�⣬�蹺��Ļ���ԭ����̼���ơ����ᣮ
�ʴ�Ϊ��̼���ơ����ᣮ
��5������Һ�л�ù������ʣ�������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
�ʴ�Ϊ����ȴ�ᾧ��
��6�������̿���п��������ֱ�Ϊxg��yg�����ݢڿ�֪MnO2��Zn�����ʵ���֮��Ϊ1��1����
| 0.7xg |
| 87g/mol |
| 0.8yg |
| 97g/mol |
�ʴ�Ϊ��1.03��
��������ʵ���Ŀ�����Ʊ�����������п�������Ҫ������������Ԫ�ء���Ԫ�س�ȥ���ݴ˿���ѧ���Թ������̵����⡢�������ʡ�������ԭ��Ӧ�������ᴿ������������ѧ����ȣ��Ѷ��еȣ��ؼ������������Ʊ�����ԭ����Ҫ��ѧ��Ҫ����ʵ�Ļ���֪ʶ�����Ӧ��֪ʶ��������������

��ϰ��ϵ�д�
�����Ŀ
