��Ŀ����
��1���£�N2H4���ֳ���������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϡ���֪��101kPaʱ��16.0gN2H4����������ȫȼ�����ɵ������ų�����312kJ��25��ʱ����д����ʾN2H4ȼ���ȵ��Ȼ�ѧ����ʽ ��
��2������----����ȼ�ϵ����20%~30%��KOH��ҺΪ�������Һ��
�����ĵ缫��Ӧʽ�� ��
�����ĵ缫��Ӧʽ�� ��
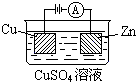
��3����ͼ��һ���绯ѧ����ʾ��ͼ��
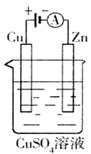
��пƬ�Ϸ����ĵ缫��Ӧ�� ��
�ڼ���ʹ���¡�����ȼ�ϵ����Ϊ�������еĵ�Դ��ͭƬ�������仯128g������һ����ȼ�ϵ�����������ı��״���µĿ��� L����������������������Ϊ20%��
��1��N2H4��l��+O2(g) == N2(g)+2H2O(g) ;��H=-624 kJ/mol
��2��2O2+4H2O+8e==8OH- CH4+10OH-��8e==CO32-+7H2O
��3��Cu2++2e=Cu 112L
����:
��1������������ʽ���Ӻ������ɵ�����ȫȼ�յ��Ȼ�ѧ����ʽN2H4��g��+O2��g��=N2��g��+2H2O��g����H=��534kJ/mol����2����طŵ�ʱ������������ԭ��Ӧ����O2����ԭ���缫��ӦʽΪO2+2H2O+4e-=4OH-������ʱ��������ˮ���ɣ���KOH��ҺŨ�ȼ�С��pH��С��


 ��1���£�N2H4���ֳ���������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϣ���֪��101kPaʱ��32.0gN2H4����������ȫȼ�����ɵ������ų�����624kJ��25��ʱ����N2H4��ȫȼ�շ�Ӧ���Ȼ�ѧ����ʽ��
��1���£�N2H4���ֳ���������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϣ���֪��101kPaʱ��32.0gN2H4����������ȫȼ�����ɵ������ų�����624kJ��25��ʱ����N2H4��ȫȼ�շ�Ӧ���Ȼ�ѧ����ʽ��