��Ŀ����
��1���£�N2H4���ֳ���������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϣ���֪��101kPaʱ��32.0gN2H4����������ȫȼ�����ɵ������ų�����624kJ��25��ʱ����N2H4��ȫȼ�շ�Ӧ���Ȼ�ѧ����ʽ�� ��
��2����-����ȼ�ϵ����һ�ּ���ȼ�ϵ�أ��������Һ��20%��30%��KOH��Һ����-����ȼ�ϵ�طŵ�ʱ��
�����ĵ缫��Ӧʽ�� ��
�����ĵ缫��Ӧʽ�� ��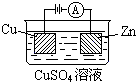
��3����ͼ��һ���绯ѧ����ʾ��ͼ��
��пƬ�Ϸ����ĵ缫��Ӧ�� ��
�ڼ���ʹ����-����ȼ�ϵ����Ϊ�������еĵ�Դ��ͭƬ�������仯128g������һ����ȼ�ϵ�����������ı�״���µĿ��� L����������������������Ϊ20%��
��4����ͳ�Ʊ��µķ���������NaClO����NH3���Ƶ��µ�ϡ��Һ���÷�Ӧ�����ӷ���ʽ�ǣ� ��
��2����-����ȼ�ϵ����һ�ּ���ȼ�ϵ�أ��������Һ��20%��30%��KOH��Һ����-����ȼ�ϵ�طŵ�ʱ��
�����ĵ缫��Ӧʽ��
�����ĵ缫��Ӧʽ��

��3����ͼ��һ���绯ѧ����ʾ��ͼ��
��пƬ�Ϸ����ĵ缫��Ӧ��
�ڼ���ʹ����-����ȼ�ϵ����Ϊ�������еĵ�Դ��ͭƬ�������仯128g������һ����ȼ�ϵ�����������ı�״���µĿ���
��4����ͳ�Ʊ��µķ���������NaClO����NH3���Ƶ��µ�ϡ��Һ���÷�Ӧ�����ӷ���ʽ�ǣ�
��������1�������ºͷ�Ӧ�ȵĹ�ϵ�������ȼ���ȣ���д������Ӧ���Ȼ�ѧ����ʽ��
��2��ȼ�ϵ���У�������Ͷ�ŵ���ȼ�ϣ�������ȼ��ʧ���ӷ���������Ӧ��
��3���ٵ��صĸ������������ӷ����õ��ӵĻ�ԭ��Ӧ��
�ڸ��ݵ缫��Ӧ����ϵ����غ�Ϳ��������������ش�
��NaClO����NH3�����Ƶ��£��������Ȼ��ƣ�
��2��ȼ�ϵ���У�������Ͷ�ŵ���ȼ�ϣ�������ȼ��ʧ���ӷ���������Ӧ��
��3���ٵ��صĸ������������ӷ����õ��ӵĻ�ԭ��Ӧ��
�ڸ��ݵ缫��Ӧ����ϵ����غ�Ϳ��������������ش�
��NaClO����NH3�����Ƶ��£��������Ȼ��ƣ�
����⣺��1����32.0g�µ����ʵ���Ϊ1mol��1molN2H4����������ȫȼ�����ɵ����ų�����624kJ���������Ȼ�ѧ��Ӧ����ʽΪ��N2H4��l��+O2��g��=N2��g��+2H2O��l����H=-624KJ/mol��
�ʴ�Ϊ��N2H4��l��+O2��g��=N2��g��+2H2O��l����H=-624KJ/mol��
��2��ȼ�ϵ���У�������Ͷ�ŵ���ȼ�ϣ�������ȼ��ʧ���ӷ���������Ӧ���ڼ��Ի����µķ�ӦʽΪ��N2H4+4OH--4e-=4H2O+N2���������������������õ��ӵĻ�ԭ��Ӧ���ڼ��Ի����£��缫��ӦʽΪ��2H2O+O2+4e-=4OH-���ʴ�Ϊ��2H2O+O2+4e-=4OH-��N2H4+4OH--4e-=4H2O+N2����
��3���ٵ��صĸ�������������ͭ���ӷ����õ��ӵĻ�ԭ��Ӧ����Cu2++2e-=Cu��
�ʴ�Ϊ��Cu2++2e-=Cu��
��ͭƬ�ϵĵ缫��ӦΪ��Cu-2e-=Cu��Cu�������仯128g�����ݵ缫��Ӧ��ת�Ƶ���Ϊ4mol�����ݷ�ӦN2H4+O2=N2+2H2O����ת�Ƶ���4molʱ���������������ʵ�����1mol������������ǿ�����������֮һ���������Ŀ����������5mol����112L��
�ʴ�Ϊ��112��
��4��NaClO����NH3�����Ƶ��µ����ӷ���ʽΪ��ClO-+2NH3=N2H4+Cl-+H2O��
�ʴ�Ϊ��ClO-+2NH3=N2H4+Cl-+H2O��
�ʴ�Ϊ��N2H4��l��+O2��g��=N2��g��+2H2O��l����H=-624KJ/mol��
��2��ȼ�ϵ���У�������Ͷ�ŵ���ȼ�ϣ�������ȼ��ʧ���ӷ���������Ӧ���ڼ��Ի����µķ�ӦʽΪ��N2H4+4OH--4e-=4H2O+N2���������������������õ��ӵĻ�ԭ��Ӧ���ڼ��Ի����£��缫��ӦʽΪ��2H2O+O2+4e-=4OH-���ʴ�Ϊ��2H2O+O2+4e-=4OH-��N2H4+4OH--4e-=4H2O+N2����
��3���ٵ��صĸ�������������ͭ���ӷ����õ��ӵĻ�ԭ��Ӧ����Cu2++2e-=Cu��
�ʴ�Ϊ��Cu2++2e-=Cu��
��ͭƬ�ϵĵ缫��ӦΪ��Cu-2e-=Cu��Cu�������仯128g�����ݵ缫��Ӧ��ת�Ƶ���Ϊ4mol�����ݷ�ӦN2H4+O2=N2+2H2O����ת�Ƶ���4molʱ���������������ʵ�����1mol������������ǿ�����������֮һ���������Ŀ����������5mol����112L��
�ʴ�Ϊ��112��
��4��NaClO����NH3�����Ƶ��µ����ӷ���ʽΪ��ClO-+2NH3=N2H4+Cl-+H2O��
�ʴ�Ϊ��ClO-+2NH3=N2H4+Cl-+H2O��
������������һ���й��Ȼ�ѧ�͵绯ѧ֪ʶ���ۺϿ���֪ʶ��Ŀ��Ҫ��ѧ�����з����ͽ��������������ѶȲ���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ

 ��1���£�N2H4���ֳ���������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϣ���֪��101kPaʱ��32.0gN2H4����������ȫȼ�����ɵ������ų�����624kJ��25��ʱ����N2H4��ȫȼ�շ�Ӧ���Ȼ�ѧ����ʽ��
��1���£�N2H4���ֳ���������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϣ���֪��101kPaʱ��32.0gN2H4����������ȫȼ�����ɵ������ų�����624kJ��25��ʱ����N2H4��ȫȼ�շ�Ӧ���Ȼ�ѧ����ʽ��