��Ŀ����
��ȸʯ��Ҫ��Cu2(OH)2CO3�������������Ļ������Ļ�����Կ�ȸʯΪԭ�Ͽ��Ʊ�CuCl2��3H2O�����������ͼ��ʾ��
��֪����ҺAֻ��Cu2����Fe2����Fe3�����ֽ������ӣ����������ӳ���ʱ��pH�����ʾ���ش��������⣺
| �������� | Fe3�� | Fe2�� | Cu2�� | |
| pH | �������� ��ʼ���� | 1.9 | 7.0 | 4.7 |
| �������� ��ȫ���� | 3.2 | 9.0 | 6.7 | |
(2)����CuO�������ǵ�����ҺpH����pH�ķ�ΧΪ________��
(3)����E��F�뱥��ʳ��ˮ��������H��Gʱ��E��FӦ��һ���Ⱥ�˳��ͨ�뱥��ʳ��ˮ�С����У�Ӧ��ͨ���������________(����ż���Ӧ���ʵĻ�ѧʽ)��
(4)����ҺC���CuCl2��3H2O����Ҫ����__________��________�����˵Ȳ�����
(5)��֪��������Cu(OH)2��Ksp��2��10��20������Cu2����2H2O
 Cu(OH)2��2H����ƽ�ⳣ��Ϊ________��
Cu(OH)2��2H����ƽ�ⳣ��Ϊ________��
(1)Cl2��H2O2
(2)3.2��pH<4.7
(3)F����NH3
(4)����Ũ������ȴ�ᾧ
(5)5��10��9
����

��ϰ��ϵ�д�
 ����������������ϵ�д�
����������������ϵ�д�
�����Ŀ
��20.00mLϡH2SO4����μ���0.10mol/L�İ�ˮ�������Һ��pH����백ˮ������仯��ͼ��ʾ�������ж���ȷ����(����) 
| A��x=20 |
| B��ϡH2SO4��Ũ��Ϊ0.10mol/L |
| C��b����2c(NH4+) = c(SO42-)> c(H+) = c(OH-) |
| D��a����c(NH4+) < 2c(SO42-) |
��ϡ��ˮ�д�����������ƽ��NH3+H2O NH3 ��H2O
NH3 ��H2O NH4++OH�����ֱ���������������ʣ���Һ��c(OH��)��α仯��(�������С�����䡱)��ƽ���ƶ�������Σ�(����������ƶ���)
NH4++OH�����ֱ���������������ʣ���Һ��c(OH��)��α仯��(�������С�����䡱)��ƽ���ƶ�������Σ�(����������ƶ���)
| ��������� | ����(NH4)2SO4���� | ����HNO3��Һ | ����KOH��Һ |
| c(OH-)�ı仯 | | | |
| ƽ���ƶ����� | | | |
���ֽⷴӦ����ѧ��ѧ�г�����һ�ַ�Ӧ���͡�
(1)��֪�ڳ����²��Ũ�Ⱦ�Ϊ0.1 mol��L��1������6����Һ��pH(C6H5OH�൱��һԪ����)��
| ���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN | C6H5ONa |
| pH | 8.8 | 9.7 | 11.6 | 10.3 | 11.1 | 11.3 |
���ֽ��������һ�����ɣ�һ�ֽ�ǿ������һ�ֽ�������ο����Է��ط�Ӧ�����ɽ�����ͽ�ǿ����Σ��磺2CH3COOH��Na2CO3=2CH3COONa��CO2����H2O���������Ƕȿ�����ͬʱ��ʾ����һ�����ɣ������Խ�ǿ�����ʷ������Ʒ�Ӧ�������ɼ��Խ��������ʡ����ոù��ɣ����ж����з�Ӧ���ܳ�������______________(����)��
A��CO2��H2O��2NaClO=Na2CO3��2HClO
B��CO2��H2O��NaClO=NaHCO3��HClO
C��CO2��H2O��C6H5ONa�D��NaHCO3��C6H5OH
D��CO2��H2O��2C6H5ONa�D��Na2CO3��2C6H5OH
E��Na2CO3��C6H5OH�D��NaHCO3��C6H5ONa
F��CH3COOH��NaCN=CH3COONa��HCN
(2)����ǰ����Ϣ�жϣ�Ũ�Ⱦ�Ϊ0.05 mol��L��1�������������ʵ���Һ�У�pH��С����________(����)����pH����________(����ֵ)��pH������________(����)��
��C6H5OH����CH3COOH����HCN����HClO
��H2SO4����HClO4
(3)һЩ���ֽⷴӦ�ķ�������ѭ�����Ĺ��ɡ�����ת�������ڸ��ֽⷴӦ��
�ٹ�ҵ�Ͻ�ʯ�����봿����Һ��Ͽ��Ƶÿ�������Һ
�ں����Ƽ�У���̼�������Һ�м��뱥��ʳ��ˮ�ɻ��С�մ���
������KCl��NaNO3�����Һ����������NaCl�������������Ӧ���ܽ�����ֽⷴӦ��������һ����___________________________________________
��KI��Һ��AgCl�����Ͻ��裬��۲쵽��������____________________________________����д����Ӧ�����ӷ���ʽ______________________________________________
ijѧ����0.100 mol��L-1��KOH����Һ�ζ�δ֪Ũ�ȵ�����,������ɷֽ�Ϊ���¼���:
| A����ȡ20.00 mL����������Һע��ྻ����ƿ,������2~3�η�̪; |
| B���ñ���Һ��ϴ�ζ���2~3��; |
| C����ʢ�б���Һ�ļ�ʽ�ζ��̶ܹ���,���ڵζ��ܼ���ʹ֮������Һ; |
| D��ȡ��KOH��Һע���ʽ�ζ������̶ȡ�0������2~3 mL; |
F.����ƿ���ڵζ��ܵ�����,�ñ�KOH��Һ�ζ����յ㲢���µζ���Һ��Ŀ̶ȡ�
�ʹ�ʵ��������:
(1)��ȷ���������˳����(����ĸ�����д)��������������������
(2)����B���������Ŀ������������������������������������������
(3)����A�������֮ǰ,�����ô�����Һ��ϴ��ƿ,��ζ��������������(�ƫ�ߡ���ƫ�͡����䡱)��
(4)�жϵ���ζ��յ��ʵ����������������������������������������������
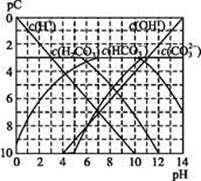
 ����ʹ����ѪҺpH������7.35~7.45�����á������õ������Һ�е�ƽ�����:������������������������������(�����ӷ���ʽ��ʾ)��
����ʹ����ѪҺpH������7.35~7.45�����á������õ������Һ�е�ƽ�����:������������������������������(�����ӷ���ʽ��ʾ)��  ������������
������������  ��������������10%��������Ƽ�ʵ��֤����ͬѧ�Ĺ۵��Ƿ���ȷ��
��������������10%��������Ƽ�ʵ��֤����ͬѧ�Ĺ۵��Ƿ���ȷ��  )="0.075" mol��L-1����t1ʱ����������ϵ�м���100 mL 0.125 mol��L-1 Na2CO3��Һ,��ʽ����˵���Ƿ��г���������
)="0.075" mol��L-1����t1ʱ����������ϵ�м���100 mL 0.125 mol��L-1 Na2CO3��Һ,��ʽ����˵���Ƿ��г���������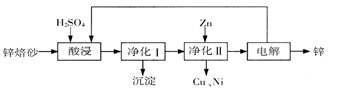
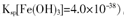 ��
��