��Ŀ����
19������������SnSO4����һ����Ҫ�������Σ��㷺Ӧ���ڶ�����ҵ��ij�о�С�����SnSO4�Ʊ�·�����£�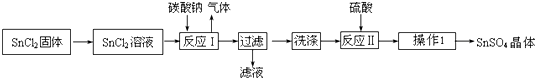
�������ϣ�
I�����������£�����ˮ��Һ����Sn2+��Sn4+������Ҫ������ʽ��
��Sn2+��������Fe2+���������ƣ�
�ش��������⣺
��1����Ԫ�������ڱ��е�λ���ǵ������ڵڢ�A�壮
��2����SnCl2��������SnCl2��Һ�ķ����ǽ�SnCl2��������Ũ���ᣬ��ˮϡ��������Ũ�ȣ��ټ�������Sn�ۣ�
��3����ӦI�õ�������SnO���÷�Ӧ�����ӷ���ʽ��Sn2++CO32-�TSnO��+CO2����
��4��ϴ��SnO�����ķ�������������м�������ˮ����û��������ˮ��ȫ�������ظ�����2-3�Σ�
��5�����������£�SnSO4����������˫��ˮȥ������������Ӧ�����ӷ���ʽ��Sn2++H2O2+2H+�TSn4++2H2O��
��6����С��ͨ�����з����ⶨ����SnCl2����Ĵ��ȣ����ʲ����뷴Ӧ����
��ȡ6.00g SnCl2�������Ƴ�100mL ��Һ��
��ȡ25.00mL��Һ������Һ�м��������FeCl3���壮
������0.10mol/L��K2Cr2O7����Һ���еζ������յ�ʱ��¼����K2Cr2O7����Һ�������
���ظ����Тڡ��������������ⶨ����K2Cr2O7����Һ��ƽ�����Ϊ25.00mL���Լ���SnCl2����Ĵ���95.00%��
���� SnCl2�ܽ�õ���Һ����̼���Ƴ��������ӣ����˵õ�����ϴ�Ӻ���������ܽ�õ���������Һ������Ũ����ȴ�ᾧ������ϴ�ӵõ����������壮
��1����ԭ�ӵĺ˵����Ϊ50����̼Ԫ������ͬһ���壬���ڢ�A�壬����ԭ��������ȥ����������Ԫ������ȷ�����ڵ����ڣ�
��2������Ϣ��֪��SnCl2��ˮ�����ɼ�ʽ�Ȼ��������������ᣬ����Sn2+ˮ�⣬Sn2+�ױ�����������Sn�۳�������ҺpH�⣬����ֹSn2+��������
��3����Ӧ��õ�������SnO��SnԪ�ػ��ϼ�Ϊ�仯�����ڷ�������ԭ��Ӧ��ͬʱ�������壬������Ϊ������̼��
��4��ϴ�ӳ����ڹ���װ���н��У�
��5�����������£�SnSO4����������˫��ˮȥ������˫��ˮ��ǿ�����ԣ���Sn2+�ױ�����ΪSn4+����������ԭΪˮ��
��6�����ݵ���ת���غ��뷽��ʽ�ɵù�ϵʽSn2+��2Fe3+��2Fe2+��$\frac{1}{3}$K2Cr2O7���ݴ˼���
��� �⣺��1����Ԫ����̼Ԫ������ͬһ���壬���ڢ�A�壬ԭ�Ӻ˵����Ϊ50����50-2-8-8-18=14����Sn���ڵ������ڣ��������ڱ��е�λ��Ϊ�������ڵڢ�A�壬
�ʴ�Ϊ���������ڵڢ�A�壮
��2������Ϣ��֪��SnCl2ˮ�⣬����SnCl2+H2O?Sn��OH��Cl+HCl���������ᣬʹ��ƽ�������ƶ�������Sn2+ˮ�⣬Sn2+�ױ�����������Sn�۳�������ҺpH�⣬����ֹSn2+����������SnCl2��������SnCl2��Һ�ķ����ǣ���SnCl2��������Ũ���ᣬ��ˮϡ��������Ũ�ȣ��ټ�������Sn�ۣ�
�ʴ�Ϊ����SnCl2��������Ũ���ᣬ��ˮϡ��������Ũ�ȣ��ټ�������Sn�ۣ�
��3����Ӧ��õ�������SnO��SnԪ�ػ��ϼ�Ϊ�仯�����ڷ�������ԭ��Ӧ��ͬʱ�������壬������Ϊ������̼�����ӷ���ʽΪ��Sn2++CO32-�TSnO��+CO2����
�ʴ�Ϊ��Sn2++CO32-�TSnO��+CO2����
��4��ϴ��SnO�����ķ��������ù���װ�ý���ϴ�ӣ���������м�������ˮ����û��������ˮ��ȫ�������ظ�����2-3�Σ�
�ʴ�Ϊ����������м�������ˮ����û��������ˮ��ȫ�������ظ�����2-3�Σ�
��5�����������£�SnSO4����������˫��ˮȥ������˫��ˮ��ǿ�����ԣ���Sn2+�ױ�����ΪSn4+����������ԭΪˮ�����ӷ���ʽΪ��Sn2++H2O2+2H+�TSn4++2H2O��
�ʴ�Ϊ��Sn2++H2O2+2H+�TSn4++2H2O��
��6����ʵ����̿�֪�������ķ�ӦΪ��2Fe3++Sn2+=Sn4++2Fe2+��6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O����
Sn2+��2Fe3+��2Fe2+��$\frac{1}{3}$K2Cr2O7
1 $\frac{1}{3}$
n 0.0250L��0.100mol/L
���n=0.0075mol��
100ml��Һ�к���SnCl2 ���ʵ���=0.0075mol��$\frac{100}{25}$=0.03mol��
SnCl2����Ĵ���=$\frac{190g/mol��0.03mol}{6.0g}$��100%=95.00%��
��SnCl2����Ĵ���Ϊ95.00%��
���� ���⿼�������ʷ���ķ�����ʵ�������ˮ�����Ӧ�ã��ζ�ʵ��ļ����жϺͼ���Ӧ�ã����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�| A�� | 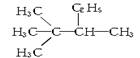 �� ��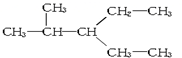 | B�� | 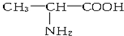 ��CH3-CH2-CH2-NO2 ��CH3-CH2-CH2-NO2 | ||
| C�� | CH3COOCH2CH3��CH3CH2COOH | D�� | C2H5-O-C2H5��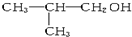 |
| A�� | v��NH3��=0.6 mol•L-1•min-1 | B�� | v��N2��=0.005 mol•L-1•s-1 | ||
| C�� | v��H2��=0.9 mol•L-1•min-1 | D�� | v��NH3��=0.02 mol•L-1•s-1 |
| A�� | C2H5OH��l��+3O2��g���T2CO2��g��+3H2O��g����H=-1367.0 kJ/mol��ȼ���ȣ� | |
| B�� | S��s��+O2��g���TSO2��g����H=-269.8kJ/mol��ȼ���ȣ� | |
| C�� | NaOH��aq��+HCl��aq���TNaCl��aq��+H2O��l����H=+57.3kJ/mol���к��ȣ� | |
| D�� | NH3•H2O��aq��+HCl��aq���TNH4Cl��aq��+H2O��l����H=-57.3kJ/mol���к��ȣ� |
�ٴӵ�ˮ����ȡ���ʵ�ʱ��������ˮ�Ҵ�����CCl4
�ڿ�����NaOH��Һ��ȥ�屽�е�������
��������KMnO4��Һ���Գ�ȥ��ϩ�л��е���Ȳ
��ʵ�������ᴿ��������������Ҵ����ɲ����ȼ���ʯ�ң����˺�������ķ�����
| A�� | �٢� | B�� | �ڢ� | C�� | �٢� | D�� | �ڢ� |
| A�� | O2��O3 | B�� | ${\;}_{1}^{1}$H��${\;}_{1}^{2}$H | ||
| C�� | CH3CH3��CH3CH2 CH3 | D�� | CH3CH2CH2CH3��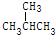 |
| A�� | �������������������NaOH��Һ��Al2O3+2OH-+3H2O�T2Al��OH��3�� | |
| B�� | ̼�����Ƶ�ˮ�⣺HCO3-+H2O?H3O++CO32- | |
| C�� | 500�桢30MPa�£���0.5mol N2��1.5mol H2�����ܱյ������г�ַ�Ӧ����NH3��g��������19.3kJ�����Ȼ�ѧ����ʽΪ��N2��g��+3H2��g��?2NH3��g����H=-38.6KJ/mol | |
| D�� | ����ı�ȼ����Ϊ-890.3kJ•mol-1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��CH4��g��+2O2��g���TCO2��g��+2H2O��1����H=-890.3kJ•mol-1 |
