��Ŀ����
5����ͬ��ͬѹ�£����и����Ȼ�ѧ����ʽ�С�H1����H2���ǣ�������| A�� | 2H2��g��+O2��g���T2H2O��l������H1 2H2��g��+O2��g���T2H2O��g������H2 | |
| B�� | 2S��g��+O2��g���T2SO2��g������H1 2S��s��+O2��g���T2SO2��g������H2 | |
| C�� | C��s��+$\frac{1}{2}$O2��g���TCO��g������H1C��s��+O2��g���TCO2��g������H2 | |
| D�� | H2��g��+Cl2��g���T2HCl��g������H1 $\frac{1}{2}$H2��g��+$\frac{1}{2}$Cl2��g���THCl��g������H2 |
���� A��Һ̬ˮ��Ϊ��̬ˮ�Ĺ��������ȹ��̣�
B���������Ϊ��̬����Ҫ����������
C��̼������ȫȼ�շ��ȶ��ڲ���ȫȼ�շŵ��ȣ�
D����ѧ��Ӧ����ʽ��ϵ���ӱ����ʱ���ֵ�ӱ���
��� �⣺A�����ʵ�ȼ�շ�Ӧ�Ƿ��ȵģ������ʱ��Ǹ�ֵ��Һ̬ˮ��Ϊ��̬ˮ�Ĺ��������ȵģ��ʡ�H1����H2����A����
B�����ʵ�ȼ�շ�Ӧ�Ƿ��ȵģ������ʱ��Ǹ�ֵ���������Ϊ��̬����Ҫ�������������ԡ�H1����H2����B����
C��̼������ȫȼ�����ɶ�����̼���ȶ��ڲ���ȫȼ������һ����̼�ŵ��ȣ���Ӧ���ʱ��Ǹ�ֵ���ʡ�H1����H2����C��ȷ��
D����ѧ��Ӧ����ʽ��ϵ���ӱ����ʱ���ֵ�ӱ����û��Ϸ�Ӧ�Ƿ��ȵģ������ʱ�ֵ�Ǹ�ֵ��2��H1=��H2����H1����H2����D����
��ѡC��
���� ���⿼�������ʷ�Ӧ�����仯�����жϣ���Ҫ�Ǹ�˹���ɵ�Ӧ�ã���������ͬ��״̬��ͬ�����ﲻͬ����Ӧ�������仯���⣬��Ŀ�ϼ�

��ϰ��ϵ�д�
�����Ŀ
13��ͼ1��Zn��Cu�γ�ԭ��أ�ijʵ����ȤС������ʵ����ڶ��鿨Ƭ����ͼ2����д����һЩ��¼���ڿ�Ƭ�ϣ������������ǣ�������


| A�� | �٢ڢ� | B�� | �ڢۢ� | C�� | �ܢݢ� | D�� | �ۢܢ� |
10��ij��������Է�������Ϊ72������������ȡ����Ӧ���õ�һ�ȴ���ֻ��һ�ֵ��ǣ�������
| A�� | CH3CH3 | B�� | 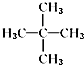 | C�� |  | D�� | 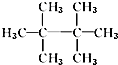 |
17����0.1mol�ģ�A��Na�� ��B�� Na2O�� ��C�� Na2O2�� ��D�� NaOH ������100mLˮ����ֽ��裬ʹ����ȫ���ܽ⣬������A��B��C��D��Һ�����ʵ�������������С�����˳������Ϊ��������
| A�� | A��B��C��D | B�� | D��A��B=C | C�� | A=B��C=D | D�� | D��A��B��C |
14������������ȷ���ǣ�������
| A�� | pH=3��pH=4�������10mL��ϣ�������Һ��pH=3.5 | |
| B�� | ��Һ��c��H+��Խ��pHֵҲԽ����Һ�����Ծ�Խǿ | |
| C�� | Һ�ȡ�Һ����Һ̬�Ȼ��ⶼ���ܵ��磬�������Ƕ����ǵ���� | |
| D�� | ���¶Ȳ���ʱ���ڴ�ˮ�м���ǿ����Һ����Ӱ��ˮ�����ӻ����� |
15��X��Y��Z��MΪ4�ֶ�����Ԫ�أ�X��Yλ��ͬ������ԭ�Ӱ뾶X��Y��Y2-��M+�ĵ��Ӳ�ṹ��ͬ��Z��X��ԭ�Ӻ���������������ͬ��Z�ĵ��ʿ��Ƴɰ뵼����ϣ�����˵������ȷ���ǣ�������
| A�� | ���� H2Y�ķ���֮��Ϊ�ܼ��ѻ� | |
| B�� | XY2��ZY2��M2Y�ľ���ֱ�����3�ֲ�ͬ���͵ľ��� | |
| C�� | ����M�ľ��������������ѻ���M����λ����8 | |
| D�� | ��Ԫ��X��Z�γɵĻ�������ֻ�����ۼ� |
