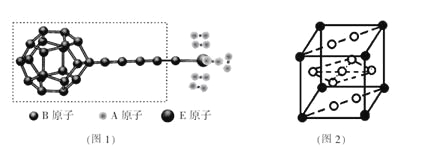
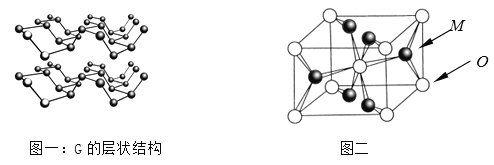
��Ŀ����
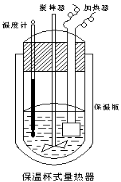
����Ŀ������ͼ��ʾ�����ȼ��У���100mL 0.50mol��L-1CH3COOH��Һ��100mL0.55mol��L-1NaOH ��Һ��ϣ��¶ȴ�25.0�����ߵ�27.7������֪���ȼƵ����ݳ��������ȼƸ�����ÿ����1���������������150.5J����-1,������Һ�ı�����Ϊ4.184J��g-1����-1����Һ���ܶȾ�����Ϊ1g��mL-1��
��1������CH3COOH�������H = kJ/mol��
��2��CH3COOH���к��ȵ�����ֵΪ56.1KJ�� mol-1������������ڣ�1���в�õ�ʵ��ֵƫ����ܵ�ԭ�� ��(������ԭ��)
��3��ʵ����NaOH������Ŀ���� ��
���𰸡���1����53.3kJ/mol����2�������ȼƵı���ƿ����Ч�����ã��������Һ��ϲ�Ѹ�٣����¶ȼƲ�����ȷ�ȴ�����ԭ�ɣ���3��ʹ�����Թ�����Ϊ���ܱ�֤CH3COOH��Һ��ȫ���кͣ��Ӷ����ʵ���ȷ�ȡ�
��������
�����������1��������H=��cm��t/n(H2O)=150.5��4.184��200]��(27.7��25)/0.05kJ��mol��1=��53.3kJ��mol��1��(2) ��ɲⶨ��ֵƫ�ͣ�Ӧ�������������ʧ��ԭ������������ȼƵı���Ч�����ã��������Һ��ϲ�Ѹ�٣����¶ȼƲ�����ȷ�ȣ�(3)ʹ�����Թ�����Ϊ���ܱ�֤CH3COOH��Һ��ȫ���кͣ��Ӷ����ʵ���ȷ�ȡ�