��Ŀ����
��Һ�п��ܺ���H+��NH4+��Mg2+��Al3+��Fe3+��CO32?��SO42?��NO3?�еļ��֡���������Ƭ��������ɫ��ζ�����壻������NaOH��Һ��������ɫ�������Ҳ����ij����������NaOH�����ʵ���֮��Ĺ�ϵ��ͼ��ʾ��������˵����ȷ����
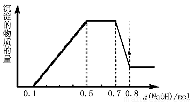
A����Һ��һ������CO32?�����ܺ���SO42?��NO3?
B���ڵμ�NaOH��Һ���ʵ���Ϊ0.5��0.7molʱ�����������ӷ�ӦΪ��Al3+ ��4OH- ��[Al(OH)4 ]-
C����Һ�е�������ֻ��H+��Mg2+��Al3+
D��n(H+)��n(NH4+)��n(Mg2+) =2��4��1
D
��������
�����������������֪������Һ��������Ƭ��������ɫ��ζ������Ϊ����������Һ���д��������ӣ���û��̼�������������ӣ����ݵ�����ԭ��֪����Һ��һ�������������������NaOH��Һ��������ɫ������˵����Һ��һ�����������ӣ����ͼ���������Һ��һ������þ���ӡ������Ӻ�����ӣ�����Һ�к������ӡ�笠����ӡ�þ���ӡ�������ʱ�������������ƣ����������ӷ�Ӧ������þ�������ӷ�Ӧ���������������������笠���Ӧ�õ�һˮ�ϰ�����������������Ӧ����ƫ�����ơ���A��������������֪����Һ��һ������CO32?��NO3?��һ������SO42?������B���������ͼ��֪���ڵμ�NaOH��Һ���ʵ���Ϊ0.5��0.7molʱ�����������ӷ�ӦΪ��NH4++OH-=NH3?H2O������C���������ͼ��֪����Һ�е�������һ����NH4+��H+��Mg2+��Al3+������D���������ͼ������������ӵ����ʵ�����0.1mol������ӵ����ʵ���Ϊ0.2mol ��þ���ӵ����ʵ�����0.05mol��n(H+)��n(NH4+)��n(Mg2+) =2��4��1����ȷ��
���㣺�������ӷ�Ӧ�����Ӽ���������ƶϡ��뻯ѧ��Ӧ��ص�ͼ���㡣

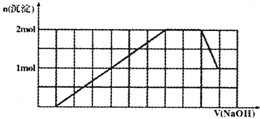 ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-��CO32-�����ӣ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ������ʵ�����NaOH��Һ������仯��ͼ����ͼ��ʾ������˵����ȷ���ǣ�������
ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-��CO32-�����ӣ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ������ʵ�����NaOH��Һ������仯��ͼ����ͼ��ʾ������˵����ȷ���ǣ�������| A��ԭ��Һ�к��е���������H+��NH4+��Mg2+��Al3+ | B��ԭ��Һ��һ������SO42-��Na+ | C��ԭ��Һ��SO42-�����ʵ�������Ϊ3.5mol | D����Ӧ����γɵ���Һ�к��е�����ΪNa2SO4 |
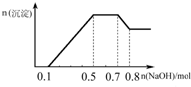 ij��Һ�п��ܺ���H+��NH4+��Mg2+��Al3+��Fe3+��CO32-��SO42-��NO3-�еļ��֣���������п����������ɫ��ζ�������壻��������NaOH��Һ��������ɫ�������Ҳ����ij��������ʵ��������NaOH�����ʵ���֮��Ĺ�ϵ��ͼ��ʾ��������˵����ȷ���ǣ�������
ij��Һ�п��ܺ���H+��NH4+��Mg2+��Al3+��Fe3+��CO32-��SO42-��NO3-�еļ��֣���������п����������ɫ��ζ�������壻��������NaOH��Һ��������ɫ�������Ҳ����ij��������ʵ��������NaOH�����ʵ���֮��Ĺ�ϵ��ͼ��ʾ��������˵����ȷ���ǣ�������| A����Һ�е�������ֻ��H+��Mg2+��Al3+ | B����Һ��n��NH4+��=0.2mol | C����Һ��һ������CO32-�����ܺ���SO42-��NO3- | D���������ӵ����ʵ���֮��n��H+����n��Al3+����n��Mg2+��=1��1��1 |
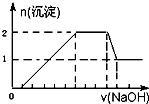 ij��ɫ��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-�������еļ��֣��������Һ�м���ijŨ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ������仯��ͼ��ʾ������˵��һ����ȷ���ǣ�������
ij��ɫ��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-�������еļ��֣��������Һ�м���ijŨ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ������仯��ͼ��ʾ������˵��һ����ȷ���ǣ�������| A��һ������H+��Mg2+��Al3+��NH4+��һ��������Na+��SO42-��Fe3+ | B��һ������H+��Al3+��NH4+��SO42-�����ܴ���Na+��Mg2+ | C����Һ��c��H+����c��Al3+����c��Mg2+��Ϊ1��1��1 | D����Һ��c��H+����c��SO42-��Ϊ2��9 |
 ��2012?���Ķ�ģ��ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-��CO32-�����ӣ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ������仯��ͼ����ͼ��ʾ������˵����ȷ���ǣ�������
��2012?���Ķ�ģ��ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-��CO32-�����ӣ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ������仯��ͼ����ͼ��ʾ������˵����ȷ���ǣ�������