��Ŀ����
��12�֣���ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ�����зų��������ɼ����к��ȡ��ش��������⣺
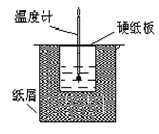
(1)��ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ���������_____________��
(2)�ձ���������ֽ����������____________________��
(3)�����ձ��ϲ���Ӳֽ�壬��õķ�Ӧ����ֵ__________(�ƫ��ƫС������Ӱ�족)��
(4)ʵ���и���60 mL 0.50 mol��L��1HCl��50 mL 0.55 mol��L��1 NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�������_________(���ȡ�����ȡ�)�������к���__________(���ȡ�����ȡ�)��
(5)����ͬŨ�Ⱥ�����İ�ˮ����NaOH��Һ��������ʵ�飬��õ��к�����ֵ��________(�ƫ��ƫС������Ӱ�족)��

(1)��ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ���������_____________��
(2)�ձ���������ֽ����������____________________��
(3)�����ձ��ϲ���Ӳֽ�壬��õķ�Ӧ����ֵ__________(�ƫ��ƫС������Ӱ�족)��
(4)ʵ���и���60 mL 0.50 mol��L��1HCl��50 mL 0.55 mol��L��1 NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�������_________(���ȡ�����ȡ�)�������к���__________(���ȡ�����ȡ�)��
(5)����ͬŨ�Ⱥ�����İ�ˮ����NaOH��Һ��������ʵ�飬��õ��к�����ֵ��________(�ƫ��ƫС������Ӱ�족)��
(12��)(1)���β�������� (2)����ʵ������е�������ʧ
(3)ƫС (4)����ȣ���ȣ�(5)ƫС
(3)ƫС (4)����ȣ���ȣ�(5)ƫС
��1����ʵ������У���Ҫ���裬���Ի�ȱ�ٻ��β����������
��2��������ʵ������У���Ҫ�����ܵļ�����������ʧ������ձ���������ֽ���������Ǽ���ʵ������е�������ʧ��
��3�������ձ��ϲ���Ӳֽ�壬��ᵼ����������ʧ�������õķ�Ӧ����ֵƫС��
��4���ı���������������Ըı䷴Ӧ�зų��������������ܸı��к��ȣ���Ϊ�к�������һ�������£�ϡ��Һ����ͼӦ����1molˮʱ�ų�������������к���ʱ����ġ�
��5�����ڰ�ˮ�д��ڵ���ƽ�⣬�����������ȵģ����Բ�õ��к�����ֵ���С��
��2��������ʵ������У���Ҫ�����ܵļ�����������ʧ������ձ���������ֽ���������Ǽ���ʵ������е�������ʧ��
��3�������ձ��ϲ���Ӳֽ�壬��ᵼ����������ʧ�������õķ�Ӧ����ֵƫС��
��4���ı���������������Ըı䷴Ӧ�зų��������������ܸı��к��ȣ���Ϊ�к�������һ�������£�ϡ��Һ����ͼӦ����1molˮʱ�ų�������������к���ʱ����ġ�
��5�����ڰ�ˮ�д��ڵ���ƽ�⣬�����������ȵģ����Բ�õ��к�����ֵ���С��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ