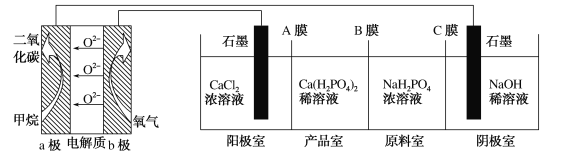
��Ŀ����
����Ŀ��NO����Ҫ��ѧ���ʡ��ش���������:
��1����ҵ��������ʱ�����Ȱ�������Ӧ�Ʊ�NO,д���÷�Ӧ�Ļ�ѧ����ʽ:_____________��
��2�������ŷ�β����NO���ô���[La0.8A0.2BCoO3+x(A.B��Ϊ����Ԫ��)]�ѳ����о�������һ���¶���,NO���ѳ����뻹ԭ��(��H2)�������Լ�����������ȱλ(��)���ܼ��̶��йء�����������:
��һ��:B4+(���ȶ�)+H2���ͼ�̬�Ľ�������(��ԭǰ������н���ԭ�ӵĸ�������)
�ڶ���:NO(g)+����NO(����̬) H1��K1
2NO(����̬)��2N(����̬)+O2(g) ��H2��K2
2N(����̬)��N2(g)+2�� ��H3��K3
2NO(����̬)��N2(g) +2O(����̬) H4��K4
2O(����̬)��O2(g)+2�� ��H5��K5
�ٵ�һ����H2��ԭB4+�õ��ͼ�̬�Ľ�������Խ��,�ڶ��η�Ӧ������Խ��,��ԭ����__________________________________��
�ڸ��ݵڶ��ο�֪��������С:NO(g)__________(�������=������)NO(����̬)��
�۸��¶���,NO�ѳ���Ӧ2NO(g)![]() N2(g)+ O2(g)��ƽ�ⳣ��K=_____(��K1��K4��K5�ı���ʽ��ʾ)��
N2(g)+ O2(g)��ƽ�ⳣ��K=_____(��K1��K4��K5�ı���ʽ��ʾ)��
��3�����ʵ���������ָ���ȶ��ĵ��ʺϳ�1mol���������ų�������(H);���ʵ������Ⱦ�Ϊ0����֪NO(g)��CO(g)��CO2(g)�������ȷֱ�Ϊ90.4kJ��mol-1��l10kJ��mol-1��393 kJ��mol-1����һ��������,NO(g)��CO(g)��Ӧ2NO(g) + 2CO(g)![]() N2(g)+2CO2(g)�ġ�H=_____��
N2(g)+2CO2(g)�ġ�H=_____��
(4)��2 L�����ܱ������г���4 mol CO��4 mol NO,������Ӧ2NO(g) +2CO(g)![]() N2(g)+ 2CO2(g),ƽ��ʱ,NO������������¶�(��)��ѹǿ(Pa)�Ĺ�ϵ��ͼ��ʾ��
N2(g)+ 2CO2(g),ƽ��ʱ,NO������������¶�(��)��ѹǿ(Pa)�Ĺ�ϵ��ͼ��ʾ��
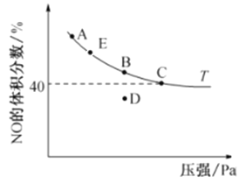
��C��NO��ƽ��ת����Ϊ______;��C����10 min�ﵽƽ�⣬��10 min��CO��ƽ����Ӧ����Ϊ________��
������ʼ������ѹǿΪ��P,��C��ʱ�÷�Ӧ��ƽ�ⳣ��Kp=____ (��ƽ���ѹ����ƽ��Ũ��,��ѹ=��ѹ�����ʵ�������)��
������D��Է�Ӧ�������µ�ͬʱ�������ʹ��ϵѹǿ��С,���´ﵽ��ƽ��״̬������________________(��ͼ��A��B��C��E��ѡ��)��
���𰸡�4NH3+5O2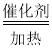 4NO+6H2O ��ԭ������н���ԭ�ӵĸ������䣬��̬���ͣ���ȱλ���࣬��Ӧ���ʼӿ� ��
4NO+6H2O ��ԭ������н���ԭ�ӵĸ������䣬��̬���ͣ���ȱλ���࣬��Ӧ���ʼӿ� �� ![]() ��K4��K5 -385.2kJ��mol-1 25% 0.05mol��L-1��min-1
��K4��K5 -385.2kJ��mol-1 25% 0.05mol��L-1��min-1 ![]() AE
AE
��������
��1�����Ĵ���������ʽΪ4NH3+5O2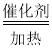 4NO+6H2O��
4NO+6H2O��
��2�������ڻ�ԭ������н���ԭ�ӵĸ������䣬��H2��ԭB4+�õ��ͼ�̬�Ľ�������Խ�࣬��ȱλ��Խ�࣬��ȱλԽ�࣬�ڶ���Խ�죬�ʴ�Ϊ����ԭ������н���ԭ�ӵĸ������䣬��̬���ͣ���ȱλ���࣬��Ӧ���ʼӿ죻
�ڵڶ��η�Ӧ���̿�֪��NO(g)����������ΪNO(����̬)��NO(����̬)���շֽ�ų�����������NO(g)��NO(����̬)���ʴ�Ϊ������
�ۢ���NO(g)+����NO(����̬) H1��K1
����2NO(����̬)��N2(g) +2O(����̬) H4��K4
����2O(����̬)��O2(g)+2�� ��H5��K5
���ݸ�˹���ɢ���2+��+���ã�2NO(g)![]() N2(g)+ O2(g) ��H��K���ɴ˿ɵ�K=
N2(g)+ O2(g) ��H��K���ɴ˿ɵ�K=![]() ��K4��K5���ʴ�Ϊ��
��K4��K5���ʴ�Ϊ��![]() ��K4��K5��
��K4��K5��
��3�����������ȵĶ����д�������Ȼ�ѧ����ʽ��
2C(g)+O2(g)=2CO(g) H1=��220 kJ��mol-1������
C(g)+O2(g)=CO2(g) H2=��393 kJ��mol-1������
N2(g)+ O2(g)=2NO(g) H3=��180.8 kJ��mol-1������
�ڡ�2-��-�۵ã�2NO(g) + 2CO(g)![]() N2(g)+2CO2(g) ��H�����ԣ���H=��393 kJ��mol-1��2+220 kJ��mol-1+180.8 kJ��mol-1=-385.2 kJ��mol-1���ʴ�Ϊ��-385.2kJ��mol-1��
N2(g)+2CO2(g) ��H�����ԣ���H=��393 kJ��mol-1��2+220 kJ��mol-1+180.8 kJ��mol-1=-385.2 kJ��mol-1���ʴ�Ϊ��-385.2kJ��mol-1��
(4)������ʽ�ⷨ���£�
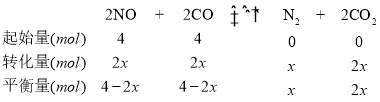
C�㣬NO���������=40%������ʽ��![]() =40%����ã�x=0.5��NO��ƽ��ת����=
=40%����ã�x=0.5��NO��ƽ��ת����=![]() ��100%=
��100%=![]() ��100%=25%��v(CO)=
��100%=25%��v(CO)=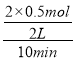 =0.05mol��L-1��min-1���ʴ�Ϊ��25%��0.05mol��L-1��min-1��
=0.05mol��L-1��min-1���ʴ�Ϊ��25%��0.05mol��L-1��min-1��
����ʼѹǿΪP��ƽ��ʱѹǿΪ![]() ����ôƽ��ʱ��NO��CO��ѹΪ��
����ôƽ��ʱ��NO��CO��ѹΪ��![]() ,N2�ķ�ѹΪ
,N2�ķ�ѹΪ![]() ��CO2��ѹΪ
��CO2��ѹΪ![]() ������Kp=
������Kp=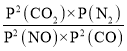 =
=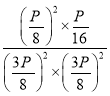 =
=![]() ��
��
��D�㷴Ӧ��δ�ﵽƽ�⣬�����ı�������ƽ���λB���ı��������൱����B��Ļ�����ƽ�ⷢ���ƶ��������¶ȣ�ƽ�������ƶ�����Сѹǿ��ƽ�������ƶ���NO����������������£���ѹ��NO�������������AE���ϣ��ʴ�ѡAE��

 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�