��Ŀ����
����Ŀ����ͼΪ��25mL 0.1mol��L1NaOH��Һ����εμ�0.2mol��L1CH3COOH��Һ��������ҺpH�ı仯���ߡ���ش�
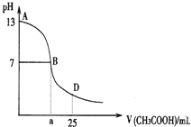
(1)B����Һ������,���˾ݴ���Ϊ,��B��ʱNaOH��CH3COOHǡ����ȫ��Ӧ,���ֿ����Ƿ���ȷ?(ѡ��ǡ���)_________.������ȷ,�����ǡ����ȫ��Ӧ�ĵ�����AB���仹��BD������?__________________(����ȷ,���ʲ���)
(2)AB����,c(OH)>c(H+),��c(OH)��c(CH3COO)��С��ϵ��_______________
A.c(OH)һ������c(CH3COO) B.c(OH)һ��С��c(CH3COO)
C.c(OH)һ������c(CH3COO) D.c(OH)���ڡ�С�ڻ����c(CH3COO)
(3)��D��ʱ,��Һ��c(CH3COO)+c(CH3COOH)_________2c(Na+)(��>������<����=��)
(4)������,��VmL��0.1000mol��L1����������Һ��μ��뵽20.00mL��0.1000mol��L1������Һ��,��ַ�Ӧ���ش���������.(������Һ����ı仯)
�������ҺpH=7,��ʱV��ȡֵ____________20.00(�>������<����=��),����Һ��c(Na+)��c(CH3COO)��c(H+)��c(OH)�Ĵ�С��ϵΪ________________________.
�����V=40.00,���ʱ��Һ��c(OH)c(H+)c(CH3COOH)=__________________mol��L1
���𰸡��� AB D = < c(Na+)=c(CH3COO)>c(H+)=c(OH) 1/30��0.033mol/L
��������
��1��NaOH��CH3COOHǡ����ȫ��Ӧ�����ɴ����ƣ�������Ϊǿ�������Σ�ˮ�����Һ�Լ��ԣ�pH��7��
��2����AB�����ڣ�����CH3COOH��NaOHǡ����ȫ��Ӧ�Լ�CH3COOH���㡢����������Һ�������ֿ����ԣ�
��3����D��ʱ��NaOH��CH3COOH��Ӧ��ʣ��CH3COOH����Һ�����Ϊ��Ũ�ȵ�CH3COOH��CH3COONa�Ļ���
��4���������NaOH��Һ�����20.00mL������ǡ����ȫ��Ӧ���ɴ����ƣ���Һ�ʼ��ԣ���Ϊ���ԣ�����������������Һ���С��20.00mL��
��1��NaOH��CH3COOHǡ����ȫ��Ӧ��NaOH+CH3COOH=CH3COONa+H20�����ɵĴ�����Ϊǿ�������Σ���Һ�Լ��ԣ�pH>7������AB֮�䣬
�ʴ�Ϊ��AB��
��2����AB�����ڣ�c��OH-��>c��H-����˵����Һ�Լ��ԣ���NaOH��CH3COOHǡ�÷�Ӧʱ���Լ��ԣ���ʱ��Һ�е�����Ϊ�����ƣ�������ˮ��̶Ƚ�С����Һ�������ԣ���c��OH-��С��c��CH3COO-������NaOH��CH3COOH��Ӧ��ʣ��NaOH����Һ��Ȼ�Լ��ԣ���ʱ��ʣ���NaOH���ܴ���c��OH-������c��CH3COO-������ȻҲ�п�����c��OH-��=c��CH3COO-����
�ʴ�Ϊ��D��
��3����D��ʱ����Ӧ��CH3COOHʣ�࣬��Һ�ɵ�Ũ�ȵ�CH3COOH��
CH3COONa��ɣ����������غ㣬��ʱ��c��CH3COO-��+c��CH3COOH��=2c��Na+����
�ʴ�Ϊ=��
��4����CH3COOH��������ʣ�����̶Ȳ���NaOH��ǿ����ʣ���ȫ���룬��Ӧ���ɵ���������ǿ�������Σ�ˮ��ʼ��ԣ�����Һ������pH=7�����ټӼ���Գ����£���V mL��0.1000molL-1����������Һ��μ��뵽20.00mL��0.1000molL-1������Һ�У���ַ�Ӧ��V<20.00mL��Һ������pH=7��c��H+��=c��OH-�������ݵ���غ�c��Na+��+c��H+��=c��CH3COO-��+c��OH-����c��H+��=c��OH-������Һ�е�����Ϊ��������Һ��ˮ�ĵ��������ģ�����c��Na+��=c��CH3COO-��>c��H+��=c��OH-����
�ʴ�Ϊ<��c��Na+��=c��CH3COO-��>c��H+��=c��OH-����
�ڸ��ݵ���غ�c��Na+��+c��H+��=c��CH3COO-��+c��OH-���������غ�c��Na+��=2[c��CH3COO-��+c��CH3COOH��]���õ�c��H+��+c��CH3COO-��+2c��CH3COOH��=c��OH-������c��OH-��-c��H+��-c��CH3COOH��=c��CH3COO-��+c��CH3COOH������Ӧ����Һ�������Ϊ60mL����c��CH3COO-��+c��CH3COOH���T0.1000mol/L��20mL��60mL=1/30mol/L��0.033mol/L��
�ʴ�Ϊ1/30��0.033��