��Ŀ����
��14�֣�ij��λ������Ϊ����ɫ���壬��ԭ���������������A��B��C��D��E����Ԫ����ɣ���ԭ�Ӹ�����Ϊl4:4:5:1:1������C��DԪ��ͬ������ԭ������DΪC�Ķ�����EԪ�ص���Χ�����Ų�Ϊ(n-1)dn��6nsl���ش��������⡣
(1)Ԫ��D�����ڱ��е�λ���� ��
��2������λ������Ļ�ѧʽΪ ��
(3)CԪ�ؿ���AԪ���γ����ֳ����Ļ������ԭ�Ӹ����ȷֱ�Ϊ1��1��l��2�����ֻ����������Ȼ��ܣ���������Ҫԭ��Ϊ ��
(4)AԪ����BԪ�ؿ��γɷ���ʽΪA2B2��ij������û�����ķ��Ӿ���ƽ��ṹ������ṹʽΪ ��
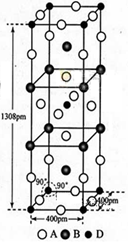
��5����֪E�ľ����ṹ����ͼ��ʾ����֪�����߳�Ϊ3��61��10-8cm����E���ܶ�Ϊ ��EDC4�������Һ������DC �Ŀռ乹���� ������Dԭ�ӵ��ӻ���������� ��
�Ŀռ乹���� ������Dԭ�ӵ��ӻ���������� ��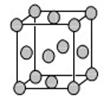
��1���� ��1�֣���A��1�֣� (2)Cu(NH3)4SO4��H2O ��2�֣�
(3)H2O��H2O2֮���γ������2�֣� (4)H-N��N-H ��2�֣�
��5��9��00g��cm��3��2�֣������������Σ�2�֣���sp3�ӻ���2�֣�
�������������EԪ�ص���Χ�����Ų�Ϊ(n-1)dn��6nsl��Ӧ��s���û������������ݺ��ع����֪����ʱd���Ӧ����ȫ����״̬����n��6��10�����n��4������EԪ�ص�ԭ��������18��10��1��29����E��ͭԪ�ء�ij��λ������Ϊ����ɫ���壬��ԭ���������������A��B��C��D��E����Ԫ����ɣ���ԭ�Ӹ�����Ϊl4:4:5:1:1������C��DԪ��ͬ������ԭ������DΪC�Ķ�������˷���������Ԫ������Ԫ������Ԫ�أ���C��O��D��S�����ݸ���λ��������ԭ�Ӹ����ȿ�֪��AӦ������Ԫ�أ�S��O��������������ʣ��1����ԭ����2����ԭ�ӽ���γ�1����ˮ����4��Bԭ�ӽ��12����ԭ�ӣ�������֮��Ϊ1:3�����Ը������ǰ�������B�ǵ�Ԫ�ء�
��1����Ԫ�������ڱ��е�λ���ǵ������ڵڢ�A�塣
��2������λ�������а��������壬��˻�ѧʽΪCu(NH3)4SO4��H2O��
��3����Ԫ������Ԫ���γɵ����ֳ�����������ˮ��˫��ˮ��������Ԫ�طǽ�����ǿ��H2O��H2O2֮���γ��������˶����ܡ�
��4����Ԫ���뵪Ԫ�ؿ��γɷ���ʽΪA2B2��ij��������N2H2���û�����ķ��Ӿ���ƽ��ṹ������8���Ӻ�2�����ȶ��ṹ��֪����Ԫ���뵪Ԫ��֮���γ�˫������Ԫ���뵪Ԫ��֮���γɵ���������ṹʽΪH-N��N-H��
��5�����ݾ����ṹ��֪�������к���ͭԭ�ӵĸ�����8��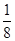 ��6��
��6��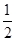 ��4�����
��4�����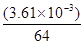 ��6.02��1023��4����æѣ�9��00g��cm��3�����������������ԭ�Ӳ����ڹ¶Ե��ӣ���۲���Ӷ�����4�����Կռ乹�������������Σ���ԭ����sp3�ӻ���
��6.02��1023��4����æѣ�9��00g��cm��3�����������������ԭ�Ӳ����ڹ¶Ե��ӣ���۲���Ӷ�����4�����Կռ乹�������������Σ���ԭ����sp3�ӻ���
���㣺����Ԫ���ƶϡ�Ԫ�����ڱ��Ľṹ��Ԫ�������ɡ��������λ������ռ乹�͡��ӻ�����Լ������ṹ�����

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д���ͼΪԪ�����ڱ���һ���֣�����Ԫ�آ١��������ڱ��е�λ�ã���Ҫ��ش��������⡣
| �� ���� | IA | | 0 | |||||
| 1 | �� | IIA | IIIA | ��A | VA | ��A | VIIA | |
| 2 | | | | | | �� | �� | |
| 3 | �� | �� | | | | �� | �� | �� |
��1����Ԫ�آ١����У���������ǿ��Ԫ����_____________����Ԫ�ط��ţ���������γɻ������Ԫ����______________����Ԫ�ط��ţ���
��2���õ���ʽ��ʾ�ߵ���̬�⻯����γɹ���__________________________________��
��3���ۡ��ܡ�����ԭ�Ӱ뾶�ɴ�С��˳����____________________����Ԫ�ط��ţ���
��4����������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬������X�ױ����ֽ⡣ijͬѧȡ5֧��С��ͬ���Թܣ�����������ʵ���Ũ�ȵ������X��Һ���ֱ��������ʵ�飬�о����������X�ֽⷴӦ���ʵ�Ӱ�죬ʵ���¼���±���ʾ��
| | ��� | ���� | ���� | ���� | |
| �¶ȣ��� | ���� | ||||
| ��һ�� | 1 | 40 | FeCl3��Һ | ���ٲ����������� | ��ͬ�����£��¶����ߣ���ѧ��Ӧ���ʼӿ� |
| 2 | 20 | A | ���������������� | ||
| 3 | 5 | FeCl3��Һ | ������������������ | ||
| �ڶ��� | 4 | t | MnO2 | ���ٲ����������� | |
| 5 | 20 | �� | ������������������ | ||
�ٵ�һ��ʵ��Ŀ���ǣ���ͬ�����£�̽��________________�Ը÷�Ӧ���ʵ�Ӱ�졣
ʵ��2�Ĵ���A��___________________��
�ڵڶ���ʵ���У�ʵ��4���¶�t��_________________��������Ӧ�Ļ�ѧ����ʽ��___________________________________________________________________________��
�ڶ���ʵ������ǣ�__________________________________________________��
�±���Ԫ�����ڱ���һ���֡��������е���ĸ�ֱ����һ�ֻ�ѧԪ�ء�
| | | | |||||||||||||||
| | | | | | a | | b | | |||||||||
| | | c | | | d | e | | ||||||||||
| | | | | | | | | f | | g | | | | | | | |
��1��a���⻯��ķ��ӹ���Ϊ ������ԭ�ӵ��ӻ���ʽΪ ��d�����������ķ��ӹ���Ϊ ������ԭ�ӵ��ӻ���ʽΪ ���÷����� ������ԡ��Ǽ��ԡ������ӡ�
��2��b��d��e����Ԫ�ص��⻯���еķе���ߵ��� ��ԭ���ǣ� ��
��3����g����ˮ�������ܽ���ˮ�У���Һ����ɫ������Ϊ������һ�ֳ���ɫ��������ӣ�д����������ӵĽṹ��ʽ(���뽫��λ����ʾ����) ��
��4��f(NH3)5BrSO4���γ���������� ����֪f 3�� ����λ����6��Ϊȷ��f�������Ľṹ���ֶ�����������������ʵ�飺�ڵ�һ����������Һ�м�BaCl2��Һʱ��������ɫ�������ڵڶ����������Һ�м���BaCl2��Һʱ�������������ڶ��������Ļ�ѧʽΪ ���������������� �� ��
��5��c���ʾ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ��
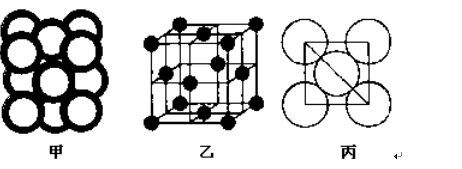
c���ʾ�����ԭ�ӵ���λ��Ϊ ������֪c��ԭ�Ӱ뾶Ϊr��NA���������ӵ�������c�����ԭ������ΪM���þ�����ܶ�Ϊ ������ĸ��ʾ����
���и��������У�������ǿ����
| A��HMnO4 | B��H2SeO3 | C��H3BO3 | D��H3PO4 |

 ��ÿ�������з�̯2����ԭ��
��ÿ�������з�̯2����ԭ�� Rx[CrCln(H2O)6��n]+xH+������0.0015 mol[CrCln(H2O)6��n]x������Һ����R-H��ȫ�������к����ɵ�H+��Ũ��Ϊ0.1200 mol��L-1NaOH��Һ25.00 mL����������ӵĻ�ѧʽΪ_______��
Rx[CrCln(H2O)6��n]+xH+������0.0015 mol[CrCln(H2O)6��n]x������Һ����R-H��ȫ�������к����ɵ�H+��Ũ��Ϊ0.1200 mol��L-1NaOH��Һ25.00 mL����������ӵĻ�ѧʽΪ_______��