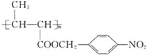
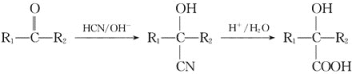
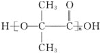
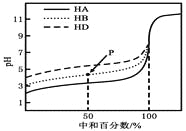
��Ŀ����
����Ŀ������˵����ȷ����( )
A.50��ʱ��ˮ��0.1mol/L����������0.1mol/LNaOH��Һ�еĵ���̶���ͬ
B.pH����7����Һһ��������
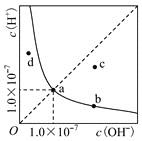
C.ˮ�����ӻ���ʾ��ˮ��Һ����ˮ�������H+��OH-Ũ�ȷ���Kw=c(H+)��c(OH-)
D.��0.06mol��L-1����������Һ��0.1mol��L-1������Һ�������ϣ��û����Һ��pH����13(���Ի�Ϲ�������Һ����仯)
���𰸡�A
��������
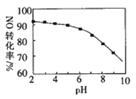
A����ͼ��ˮ�ĵ������������ã����������ͬ����ͼ��ˮ�ĵ��������������ͬ���ݴ˷����жϣ�
B��������pH=7����Һ�����ԣ�
C��ˮ�����ӻ��е�c(H+)��c(OH-)ָ������Һ��H+��OH-����Ũ�ȣ�
D����0.06mol��L-1����������Һ��0.1mol��L-1������Һ�������ϣ����������Һ�Լ��ԣ�����������������ȼ����c(OH-)����δ������Ӧ�������¶ȣ�ˮ�����ӻ�Kw��ȷ���������Һ��c(H+)��pH�����㡣
A����ͼ��ˮ�ĵ������������ã����������ͬ����ͼ��ˮ�ĵ��������������ͬ�����50��ʱ��ˮ��0.1mol/L����������0.1mol/LNaOH��Һ�еĵ���̶���ͬ��A����ȷ��
B��ֻ���ڳ����£�pH=7����Һ�����ԣ��������¶��£�pH=7����Һ���ܳ����ԣ�Ҳ���ܳʼ��ԣ�B�����
C��ˮ�����ӻ��е�c(H+)��c(OH-)ָ������Һ��H+��OH-����Ũ�ȣ�������ָˮ������ģ�C�����
D����0.06mol��L-1����������Һ��0.1mol��L-1������Һ�������ϣ����������Һ�Լ��ԣ���c(OH-)=![]() =0.01mol/L����δ������Ӧ�������¶ȣ�ˮ�����ӻ�Kw��ȷ���������Һ��c(H+)��pH�����㣬D�����
=0.01mol/L����δ������Ӧ�������¶ȣ�ˮ�����ӻ�Kw��ȷ���������Һ��c(H+)��pH�����㣬D�����
��ѡA��

 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д�