��Ŀ����
12��ij����Һֻ���±������еļ��֣�������ˮ�ĵ���������ˮ�⣩���Ҹ������ӵ����ʵ���Ũ�Ⱦ�Ϊ0.1mol•L-1��| ������ | K+ NH4+ Fe2+ Mg2+ Al3+ Cu2+ |
| ������ | OH- Cl- AlO2- CO32- SO42- SiO32- |
����˵����ȷ���ǣ�������
| A�� | ԭ��Һ��ֻ���� NH4+��Fe2+��Cl-��SO42- | |
| B�� | �ɳ���A�ƶ�ԭ��Һ��һ������SO42- | |
| C�� | ��ҺA�п��ܺ��� K+��Al3+��Cl- | |
| D�� | ����B��һ������Mg��OH��2 |
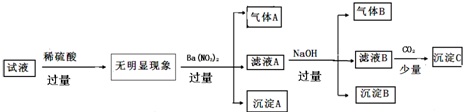
���� ��Һ�������ϡ���ᷴӦû����������˵��ԭ��Һ��һ��������SiO32-����������������Һ�м������ᱵ��Һ�õ�������A����ҺA�ͳ���A������Aֻ��ΪNO��ԭ��Һ��һ������Fe2+����һ��������OH-��AlO2-��CO32-������BΪ���ᱵ�����ڼ�����ϡ���ᣬ��ȷ��ԭ��Һ���Ƿ���SO42-����ҺA�м������NaOH��Һ�õ�����B����ҺB�ͳ���B������BΪ��������ԭ��Һ��һ������NH4+���������ӵ����ʵ���Ũ�Ⱦ�Ϊ0.1mol•L-1���Ѿ�ȷ�����е�������ΪFe2+��NH4+����������ֻ�ܺ���Cl-��SO42-����ϵ���غ��֪��Һ��һ��������K+��Mg2+��Al3+��Cu2+�����Գ���BΪ������������ҺB�к�������������ͨ�������̼�������˳���C̼�ᱵ���ݴ˽����жϣ�
��� �⣺����ʵ�������֪����Һ�������ϡ���ᷴӦ����������˵��ԭ��Һ��һ��������SiO32-����������������Һ�м������ᱵ��Һ�õ�������A����ҺA�ͳ���A������Aֻ��ΪNO��ԭ��Һ��һ������Fe2+����һ��������OH-��AlO2-��CO32-������BΪ���ᱵ�����ڼ�����ϡ���ᣬ��ȷ��ԭ��Һ���Ƿ���SO42-����ҺA�м������NaOH��Һ�õ�����B����ҺB�ͳ���B������BΪ��������ԭ��Һ��һ������NH4+���������ӵ����ʵ���Ũ�Ⱦ�Ϊ0.1mol•L-1���Ѿ�ȷ�����е�������ΪFe2+��NH4+����������ֻ�ܺ���Cl-��SO42-����ϵ���غ��֪��Һ��һ��������K+��Mg2+��Al3+��Cu2+�����Գ���BΪ������������ҺB�к�������������ͨ�������̼�������˳���C̼�ᱵ��
A�����ݷ�����֪��ԭ��Һ��һ�����ڵ�����ΪNH4+��Fe2+��Cl-��SO42-����A��ȷ��
B�����ڿ�ʼʱ������ϡ���ᣬͨ������A���ж�ԭ��Һ���Ƿ�����������ӣ���B����
C����ҺA�к���Cl-����һ��������K+��Al3+����C����
D�����ݷ�����֪������BΪ̼�ᱵ��ԭ��Һ��һ��������þ���ӣ���D����
��ѡA��
���� ���⿼���˳������ӵļ��鷽������Ŀ�Ѷ��еȣ���ȷ�������ӵ�����Ϊ���ؼ���ע�����ճ������ӵļ��鷽�������ݵ���غ��ж����ӵ�������Ϊ�״��㣮

 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�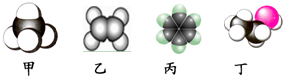
| A�� | ���Ǽ��飬������ʹ����KMnO4��Һ��ɫ | |
| B�� | ������ϩ����ϩ������ˮ����ȡ����Ӧʹ��ˮ��ɫ | |
| C�� | ���DZ������ڿ�����ȼ��ʱ��������Ũ�̵Ļ��� | |
| D�� | �������ᣬһ�������������ܸ��Ҵ�����ȡ����Ӧ |
| A�� | �������������еı�������ʧȥ���� | |
| B�� | ���ǵ���ȵ������� | |
| C�� | �Ʊ�����ú���У�����Ϊ�Ƶ��ܶȱ�ú��С | |
| D�� | ���ڿ�����ȼ�շ�����ɫ���� |
| A�� | �ò�˿պȡNa2CO3��Һ�ھƾ����������գ��۲쵽��ɫ���� | |
| B�� | ������������ˮ�� | |
| C�� | ľ��պȡŨ�������ɫ | |
| D�� | ��ˮ���μ��˷�̪��Һ���Ͱ�������Ȫʵ�� |
| A�� | pH=7����Һ | |
| B�� | Kw=c��H+��•c��OH-��=1.0��10-14����Һ | |
| C�� | c��H+��=c��OH-�� | |
| D�� | pH=3������pH=11�ļ�������Ϻ����Һ |
| A�� | ��������Ͷ�뵽һ������ϡ�����У���ַ�Ӧ��ȡ�ϲ���Һ���Թ��У��μ�KSCN��Һ����Һ��Ѫ��ɫ | |
| B�� | �Ʊ�����������ʱ��������������Һ�еμ�����������Һ���ӱ߽��裬�����Ƶð�ɫ������������ | |
| C�� | �����ש�е��������ɷ֣����ש��ĩ�м������ᣬ��ַ�Ӧ��ȡ�ϲ���Һ���Թ��У��μ�KSCN��Һ2��3�μ��� | |
| D�� | FeOͶ��ϡH2SO4��ϡHNO3�о��õ�dz��ɫ��Һ |
 ��
�� ���������ᣨH2N2O2����һ�ֶ�Ԫ�ᣬ��������N2O���壮
���������ᣨH2N2O2����һ�ֶ�Ԫ�ᣬ��������N2O���壮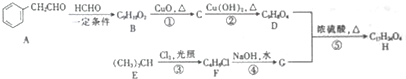

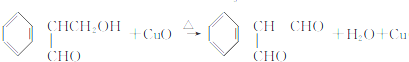
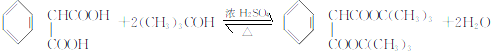
 ��
��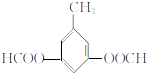 �������������칹��•
�������������칹��• �ĺϳ�·�ߣ�
�ĺϳ�·�ߣ�