��Ŀ����
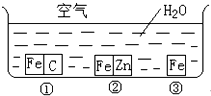
 ��η�ֹ������ʴ�ǹ�ҵ���о����ص����ݣ�Ϊ�о�������ʴ��ijͬѧ����̽��ʵ�飬����ͼ��ʾ�������ڢ١��ڡ������ֲ�ͬ�Ļ����У���ش�
��η�ֹ������ʴ�ǹ�ҵ���о����ص����ݣ�Ϊ�о�������ʴ��ijͬѧ����̽��ʵ�飬����ͼ��ʾ�������ڢ١��ڡ������ֲ�ͬ�Ļ����У���ش���1��������ʴ�������ɿ쵽����˳����
�٢ۢ�
�٢ۢ�
������ţ�����2�������ڢٻ���ʱ��������Ӧʽ
O2+2H2O+4e-=4OH-
O2+2H2O+4e-=4OH-
��3������Ϊ���¸�����Ʒѡ���ʵ��ķ��ⷽ����
A�����������������
Ϳ��֬
Ϳ��֬
B����ˮ�е��ִ�
��ӵ�Դ����������������������������������
��ӵ�Դ����������������������������������
��������ͼ�Т�����̼�Ӵ�����ʴ������ʴ��������п�Ӵ��γ�ԭ��ط�Ӧ��п�������ã������������۸�ʴ��ѧ��ʴ�����ʱȵ绯ѧ��ʴ�����Դ˽����⣮
����⣺��1��ͼ�Т�����̼�Ӵ�����ʴ������ʴ��������п�Ӵ��γ�ԭ��ط�Ӧ��п�������ã������������۸�ʴ��ѧ��ʴ�����ʱȵ绯ѧ��ʴ������������ʴ�������ɿ쵽����˳���Ǣ٢ۢڣ��ʴ�Ϊ���٢ۢڣ�
��2�������ڢٻ���ʱ������������ԭ��Ӧ��Ϊ������ʴ��������ӦʽΪO2+2H2O+4e-=4OH-���ʴ�Ϊ��O2+2H2O+4e-=4OH-��
��3��A��Ϊ��ֹ���г������������ָ�ʴ����Ϳ��֬���ʴ�Ϊ��Ϳ��֬��
B��Ϊ��ֹ��ˮ�е��ִ�����ʴ��������ӵ�Դ�������������������������������������ʴ�Ϊ����ӵ�Դ������������������������������������
��2�������ڢٻ���ʱ������������ԭ��Ӧ��Ϊ������ʴ��������ӦʽΪO2+2H2O+4e-=4OH-���ʴ�Ϊ��O2+2H2O+4e-=4OH-��
��3��A��Ϊ��ֹ���г������������ָ�ʴ����Ϳ��֬���ʴ�Ϊ��Ϳ��֬��
B��Ϊ��ֹ��ˮ�е��ִ�����ʴ��������ӵ�Դ�������������������������������������ʴ�Ϊ����ӵ�Դ������������������������������������
���������⿼������ĸ�ʴ���������Ŀ�ѶȲ���ע����ս�����ʴ��ԭ�������յ�ⷽ��ʽ����д�Լ�����������ʴ�Ĵ�ʩ��ѧϰ��ע����ۣ�

��ϰ��ϵ�д�
�����Ŀ
��η�ֹ������ʴ�ǹ�ҵ���о����ص����ݣ�Ϊ�о�����ʴ��Ӱ�����أ�ijͬѧ��������̽��ʵ�飺
�ش��������⣺
��1������ʵ���з����˵绯ѧ��ʴ���ǣ���ʵ����ţ� ���ڵ绯ѧ��ʴ�У�������Ӧ�� ��������Ӧ�� ��
��2���ɸ�ʵ���֪������Ӱ������ʴ���ʵ������� ��
��3��Ϊ��ֹ������ʴ����ҵ���ձ���õķ����� �������ַ�������
| ��� | ���� | ʵ������ |
| 1 | �����½���˿���ڸ��������һ���� | �������˿������Ȼ���� |
| 2 | �����½���˿���ڳ�ʪ������һСʱ | ��˿������Ȼ���� |
| 3 | �����½���˿���ڳ�ʪ�Ŀ�����һ���� | ��˿�����ѱ�ûҰ� |
| 4 | ����ʪ����˿���ڳ��µ���������һСʱ | ��˿�������ԻҰ� |
| 5 | ����ʪ����˿���ڸ��ڳ��µ���������һСʱ | ��˿�����ѱ�ûҰ� |
| 6 | �������Ȼ�����Һ����˿���ڸ��ڳ��µ���������һСʱ | ��˿����Ұ��̶ȱ�ʵ��5���� |
��1������ʵ���з����˵绯ѧ��ʴ���ǣ���ʵ����ţ�
��2���ɸ�ʵ���֪������Ӱ������ʴ���ʵ�������
��3��Ϊ��ֹ������ʴ����ҵ���ձ���õķ�����
 ��η�ֹ������ʴ�ǹ�ҵ���о����ص����ݣ�Ϊ�о�������ʴ��ijͬѧ����̽��ʵ�飬��ͼ��ʾ�������ڢ١��ڡ������ֲ�ͬ�Ļ����У���ش�
��η�ֹ������ʴ�ǹ�ҵ���о����ص����ݣ�Ϊ�о�������ʴ��ijͬѧ����̽��ʵ�飬��ͼ��ʾ�������ڢ١��ڡ������ֲ�ͬ�Ļ����У���ش�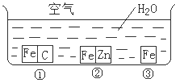 ��η�ֹ������ʴ�ǹ�ҵ���о����ص����ݣ�Ϊ�о�������ʴ��ijͬѧ����̽��ʵ�飬��ͼ��ʾ�������ڢ١��ڡ������ֲ�ͬ�Ļ����У���ش�
��η�ֹ������ʴ�ǹ�ҵ���о����ص����ݣ�Ϊ�о�������ʴ��ijͬѧ����̽��ʵ�飬��ͼ��ʾ�������ڢ١��ڡ������ֲ�ͬ�Ļ����У���ش�