题目内容
【题目】【2016届天水市一中期末】钠、钾的碘化物在生产和科学实验中有十分重要的应用。工业利用碘、NaOH和铁屑为原料可生产碘化钠,其生产流程如图所示:
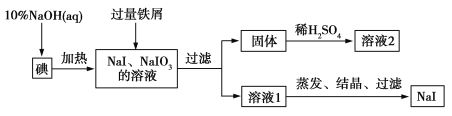
(1)NaOH溶液和碘反应时需要严格控制温度,如果温度过低,会生成碘的低价副产品NaIO。若NaOH溶液和碘反应时所得溶液中IO![]() 与IO-的物质的量之比为1∶1,则该反应的离子方程式为______ 。
与IO-的物质的量之比为1∶1,则该反应的离子方程式为______ 。
(2)生产流程中加入过量铁屑的目的是__________________,过滤所得固体中除剩余铁屑外,还有红褐色固体,则加入铁屑时发生反应的化学方程式是________ __________。
(3)溶液2中除含有H+外,一定含有的阳离子是______ ____________;试设计实验证实该金属阳离子的存在:__________________________________。
(4)溶液2经一系列转化可以得到草酸亚铁晶体(FeC2O4·2H2O),称取3.60 g草酸亚铁晶体(相对分子质量是180)用热重法对其进行热分解,得到剩余固体的质量随温度变化的曲线如图所示:
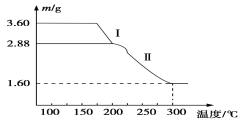
①分析图中数据,根据信息写出过程I发生的化学方程式:____________ ______。
②300℃时剩余固体只有一种且是铁的氧化物,试通过计算确定该氧化物的化学式:________ ________。
【答案】(1)4I2+8OH-===IO![]() +6I-+4H2O+IO-
+6I-+4H2O+IO-
(2)还原IO![]() (或使IO
(或使IO![]() 转化为I-)(合理即可)
转化为I-)(合理即可)
NaIO3+2Fe+3H2O===NaI+2Fe(OH)3↓
(3)Fe2+ 取少量试样溶液于试管中,滴入少量酸性KMnO4溶液,若KMnO4溶液褪色,证明存在Fe2+(或者加入K3[Fe(CN)6]产生蓝色沉淀)
(4)①FeC2O4·2H2O![]() FeC2O4+2H2O↑
FeC2O4+2H2O↑
②Fe2O3
【解析】(1)NaOH溶液和碘反应时所得溶液中IO![]() 与IO-的物质的量之比为1∶1,若均为1mol,则由电子守恒可知,生成碘离子的物质的量为6mol,结合电荷守恒及原子守恒可知离子反应为:4I2+8OH-===IO
与IO-的物质的量之比为1∶1,若均为1mol,则由电子守恒可知,生成碘离子的物质的量为6mol,结合电荷守恒及原子守恒可知离子反应为:4I2+8OH-===IO![]() +6I-+4H2O+IO-。(2)加入铁屑的目的是还原IO
+6I-+4H2O+IO-。(2)加入铁屑的目的是还原IO![]() ,使IO
,使IO![]() 转化为I-,加入铁屑的方程式为NaIO3+2Fe+3H2O===NaI+2Fe(OH)3↓。(3)固体中除剩余的铁屑外,还有红褐色固体,加入硫酸得到的溶液2中除含有H+外,固体完全溶解,一定含有Fe2+;证明含有亚铁离子的方法为取少量试样溶液于试管中,滴入少量酸性KMnO4溶液,若KMnO4溶液褪色,证明存在Fe2+(或者加入K3[Fe(CN)6]产生蓝色沉淀)。(4)①3.60 g草酸亚铁晶体,物质的量为0.2mol,过程I使其质量减少3.60-2.88=0.72克,恰好为0.4mol水的质量,则过程I发生的反应方程式:FeC2O4·2H2O
转化为I-,加入铁屑的方程式为NaIO3+2Fe+3H2O===NaI+2Fe(OH)3↓。(3)固体中除剩余的铁屑外,还有红褐色固体,加入硫酸得到的溶液2中除含有H+外,固体完全溶解,一定含有Fe2+;证明含有亚铁离子的方法为取少量试样溶液于试管中,滴入少量酸性KMnO4溶液,若KMnO4溶液褪色,证明存在Fe2+(或者加入K3[Fe(CN)6]产生蓝色沉淀)。(4)①3.60 g草酸亚铁晶体,物质的量为0.2mol,过程I使其质量减少3.60-2.88=0.72克,恰好为0.4mol水的质量,则过程I发生的反应方程式:FeC2O4·2H2O![]() FeC2O4+2H2O↑;②草酸亚铁晶体中的铁元素的质量为3.6×56/180=1.12g,草酸亚铁晶体中铁元素完全转化到氧化物中,氧化物中氧元素的质量为1.60-1.12=0.48g,铁元素和氧元素的物质的量为1.12/56:0.48/16=2:3,则为Fe2O3
FeC2O4+2H2O↑;②草酸亚铁晶体中的铁元素的质量为3.6×56/180=1.12g,草酸亚铁晶体中铁元素完全转化到氧化物中,氧化物中氧元素的质量为1.60-1.12=0.48g,铁元素和氧元素的物质的量为1.12/56:0.48/16=2:3,则为Fe2O3