��Ŀ����
����Ŀ��������N2��O2������ӦN2(g)+O2(g)![]() 2NO(g)���ǵ�������β���к���NO��ԭ��֮һ��
2NO(g)���ǵ�������β���к���NO��ԭ��֮һ��
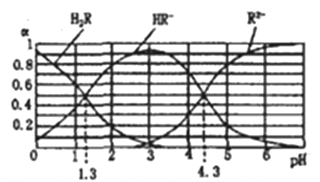
(1)��ͼ��T1��T2���ֲ�ͬ�¶��£�һ������NO�����ֽ������N2�����������ʱ��仯��ͼ�����ݴ��жϷ�ӦN2(g)+O2(g)![]() 2NO(g)Ϊ_______(��������������������)��Ӧ��
2NO(g)Ϊ_______(��������������������)��Ӧ��

(2)2000��ʱ���ݻ�Ϊ2L���ܱ������г���10molN2��5molO2���ﵽƽ���NO�����ʵ���Ϊ2mol����˷�Ӧ��ƽ�ⳣ��K=____�����¶��£�����ʼʱ�����������г���N2��O2��Ϊ1mol����ﵽƽ���N2��ת����Ϊ______��
(3)��������װ����װ�к�Pd������Ĵ����������ڴ�������������������õĻ�����ͼ��ʾ��д����仯���ܻ�ѧ��Ӧ����ʽ:__________��
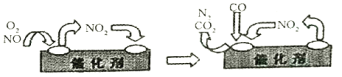
(4)Ϊ��������β����NOx���ŷ���������CxHy(��)����ԭNOx���������������Ⱦ��������
CH4(g)+4NO2(g)=4NO(g)+CO2(g)+2H2O(g) ��H=-574kJ/mol
CH4(g)+4NO(g)=2N2(g)+CO2(g)+2H2O(g) ��H2
��16gCH4��ԭNO2��N2���ų�����867kJ������H2=_______��
���𰸡� ���� 1/9��0.11 1/7��14.3% 2NO��O2��4CO![]() 4CO2��N2 ��1160 kJ��mol-1
4CO2��N2 ��1160 kJ��mol-1
��������(1)�������ȹ���ƽ��ֵ����ԭ���жϵ�T1��T2��С���ٸ���ƽ��ʱ��������������ж��¶ȶ�ƽ���Ӱ�죻
(2)ƽ�ⳣ��Ϊ����Ũ��ϵ�����ݵij˻��뷴Ӧ��Ũ��ϵ�����ݵij˻��ıȣ������¶Ȳ��䣬ƽ�ⳣ�����䣬��������ʽ�����Ӧ������������ʵ����ı仯����ƽ��ʱ��Ӧ������������ʵ���������ƽ�ⳣ������ʽ���δ֪���������ת���ʣ�
(3)����ͼ���жϳ���Ӧ����Ȼ����ƽд������ʽ��
(4)���ݸ�˹�����Ƚ���������ʽ��ӵü���ת��Ϊ�����ķ���ʽ�����ݼ���������Ĺ�ϵ���㷴Ӧ�ȡ�
(1)����ͼ���жϣ�T2�����ȵ���ƽ�⣬��Ӧ���ʴ��¶Ƚϸߣ����¶����ߣ����������������С��˵�������¶�ƽ��������Ӧ�ƶ��������¶������ȷ�����У�������ӦΪ���ȷ�Ӧ���ʴ�Ϊ�����ȣ�
(2)��������ʽ�����Ӧ������������ʵ����ı仯����ƽ��ʱ��Ӧ������������ʵ�����
N2(g)+O2(g)2NO(g)��
��ʼ(mol/L)��5 2.5 0
��Ӧ(mol/L)��0.5 0.5 1
ƽ��(mol/L)��4.5 2 1
����ƽ�ⳣ��k=![]() =
=![]() =
=![]() ��
��
��N2ת�������ʵ���Ϊxmol����
N2(g)+O2(g)2NO(g)��
��ʼ(mol)��1 10
��Ӧ(mol)��x x 2x
ƽ��(mol)��1-x 1-x2x
����ƽ�ⳣ��k=![]() =
=![]() =
=![]() �����x=
�����x=![]() ��N2��ת����Ϊ
��N2��ת����Ϊ![]() ��100%=14.3%���ʴ�Ϊ��
��100%=14.3%���ʴ�Ϊ��![]() ��14.3%��
��14.3%��
(3)����ͼ���֪��NO2Ϊ�м�����Ӧ��ΪNO��O2��CO������ΪCO2��N2����Ӧ����ʽΪ2NO+O2+4CO![]() 4CO2+N2���ʴ�Ϊ��2NO+O2+4CO
4CO2+N2���ʴ�Ϊ��2NO+O2+4CO![]() 4CO2+N2��
4CO2+N2��
(4)��CH4(g)+4NO2(g)=4NO(g)+CO2(g)+2H2O(g)��H1=-574kJmol-1����CH4(g)+4NO(g)=2N2(g)+CO2(g)+2H2O(g)��H2�����ݸ�˹���ɣ�����+�ڵ���2CH4(g)+4NO2(g)=2N2(g)+2CO2(g)+4H2O(g)��H=-574kJmol-1+��H2=867kJmol-1��2�������H2=-1160kJmol-1���ʴ�Ϊ��-1160kJmol-1��

����Ŀ��SOCl2��һ����Ҫ�Ȼ�����﮵�ص��������ϣ�������ˮ�������ε���ˮ����
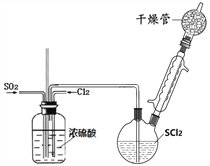
����һ��ģ���Ʊ�SOCl2װ������ͼ��ʾ���г֡�����װ����ȥ����˫��ƿ�м���25.4mL SCl2��ͨ�봿������Cl2��SO2������壬ˮԡ�����·�����ӦCl2+SO2+SCl2=2SOCl2���������ʵIJ����������±���ʾ��
�ܶ�/ g�qmL-1 | �۵�/�� | �е�/�� | ��ע | |
SCl2 | 1.62 | -122 | 59 | 40���ֽ�����ˮ�ֽ� |
SOCl2 | 1.64 | -104.5 | 76 | 76���ֽ�����ˮ�ֽ� |
��1��ϴ��ƿ�������ϲ��������___________���á�
��2��������м�ʯ������__________��
��3��ʵ������ȡSO2װ�ú�ҩƷӦѡ��____��
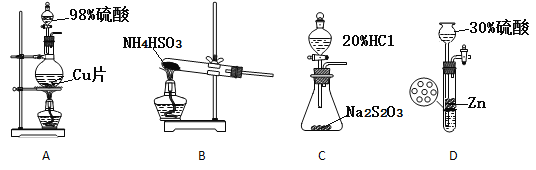
����˫��ƿ��ëϸ�����ܺ�����ͷ�����Ӽ�ѹ����װ�ã��Դֲ�Ʒ��ѹ������ʣ��Һ�������ѹ�����ò�Ʒ
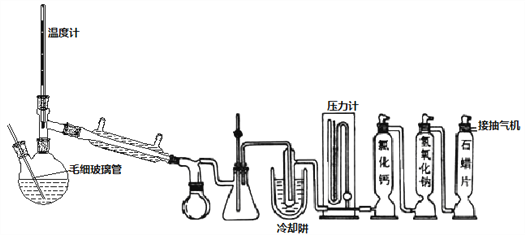
��4����������ͨ�������������ԭ����____��
��5��ëϸ������C������____________������ˮԡ���ȵ��¶�Ӧ������____�����£��������ò�Ʒ25.4mL����SOCl2����Ϊ__________��