��Ŀ����
��1����֪ij��Ӧ�ĸ�����Ũ���������£�aA��g��+bB��g�� 2C��g��
2C��g��
��ʼŨ�ȣ�mol/L����1.5 1.0 0
2sĩŨ�ȣ�mol/L����0.9 0.8 0.4
���a=
��2S��B�ķ�Ӧ����=
��2����̼������Ƭ��200mL 1.5mol/L��ϡ������ɵ�ԭ��
����
�ܴ�ʱ��Һ��H+�����ʵ���Ũ��Ϊ
��3����2.3g�����Ʒ���������m g��ˮ��D2O���У���ȫ��Ӧ��������Һ�����ʵ�����������
��100%
��100%���ú�m�Ĵ���ʽ��ʾ��
 2C��g��
2C��g����ʼŨ�ȣ�mol/L����1.5 1.0 0
2sĩŨ�ȣ�mol/L����0.9 0.8 0.4
���a=
3
3
��b1
1
����2S��B�ķ�Ӧ����=
0.1mol/��L?S��
0.1mol/��L?S��
����2����̼������Ƭ��200mL 1.5mol/L��ϡ������ɵ�ԭ��
��Ҫ�˿�
��Ҫ�˿�
���У�����̼���ϲ�������3.36L����״����ʱ��������
1.806��1023
1.806��1023
������ͨ���˵��ߣ���NA=6.02��1023���ܴ�ʱ��Һ��H+�����ʵ���Ũ��Ϊ
1.5mol/L
1.5mol/L
����������Һ����仯������3����2.3g�����Ʒ���������m g��ˮ��D2O���У���ȫ��Ӧ��������Һ�����ʵ�����������
| 4.1 |
| 2.1+m |
| 4.1 |
| 2.1+m |
��������1���������A��B��Ũ�ȱ仯����Ũ�ȱ仯��֮�ȵ��ڻ�ѧ������֮�ȣ��ɴ�ȷ��a��b��ֵ��
������v=
����v��B����
��2����������ԭ��Ӧ�е���ת����Ŀ��Ϊ������ͨ���ĵ��ӣ�
�ܸ������ɵ�����������μӷ�Ӧ��H+�����ʵ������������Һ��H+�����ʵ���Ũ�ȱ仯����ԭϡ������Һ��H+�����ʵ���Ũ�ȼ�ȥ��Һ��H+�����ʵ���Ũ�ȱ仯������Ϊ������Һ��H+�����ʵ���Ũ�ȣ�
��3������Naԭ���غ㣬���NaOD����������Һ������=�Ƶ�����+��ˮ������-���ɵ���������������������������������㣮
������v=
| ��c |
| ��t |
��2����������ԭ��Ӧ�е���ת����Ŀ��Ϊ������ͨ���ĵ��ӣ�
�ܸ������ɵ�����������μӷ�Ӧ��H+�����ʵ������������Һ��H+�����ʵ���Ũ�ȱ仯����ԭϡ������Һ��H+�����ʵ���Ũ�ȼ�ȥ��Һ��H+�����ʵ���Ũ�ȱ仯������Ϊ������Һ��H+�����ʵ���Ũ�ȣ�
��3������Naԭ���غ㣬���NaOD����������Һ������=�Ƶ�����+��ˮ������-���ɵ���������������������������������㣮
�����1��������Ŀ���ݿ�֪��A��Ũ�ȱ仯��Ϊ1.5mol/L-0.9mol/L=0.6mol/L��B��Ũ�ȱ仯��Ϊ1.0mol/L-0.8mol/L=0.2mol/L��C��Ũ�ȱ仯��Ϊ0.4mol/L��
Ũ�ȱ仯��֮�ȵ��ڻ�ѧ������֮�ȣ�����a��b��2=0.6mol/L��0.2mol/L��0.4mol/L=3��1��2������a=3��b=1��
�ʴ�Ϊ��a=3 b=1
��2S��B�ķ�Ӧ����v��B��=
=0.1mol/��L?S��
�ʴ�Ϊ��0.1mol/��L?S��
��2����̼���ϲ�������3.36LΪ��������Ӧת�Ƶĵ��ӵ����ʵ���Ϊ
��2=0.3mol�����Ե�����ͨ���ĵ�����ĿΪ0.3mol��6.02��1023mol-1=1.806��1023��
�ʴ�Ϊ��1.806��1023
�ܸ���Hԭ���غ��֪���μӷ�ӦH+�����ʵ���Ϊ
��2=0.3mol��H+�����ʵ���Ũ�ȱ仯����c��H+��=
=1.5mol/L������Һ��H+�����ʵ���Ũ��c��H+��=1.5mol/L��2-1.5mol/L=1.5mol/L��
�ʴ�Ϊ��1.5mol/L
��3��n��Na��=
=0.1mol
����Naԭ���غ�n��NaOD��=n��Na��=0.1mol������m��NaOD��=0.1mol��41g/mol=4.1g��
����ת�Ƶ����غ�n��D2��=
n��Na��=
��0.1mol=0.05mol������m��D2��=0.05mol��4g/mol=0.2g��
��Һ�����ʵ���������Ϊ
��100%=
��100%
�ʴ�Ϊ��
��100%
Ũ�ȱ仯��֮�ȵ��ڻ�ѧ������֮�ȣ�����a��b��2=0.6mol/L��0.2mol/L��0.4mol/L=3��1��2������a=3��b=1��
�ʴ�Ϊ��a=3 b=1
��2S��B�ķ�Ӧ����v��B��=
| 0.2mol/L |
| 2s |
�ʴ�Ϊ��0.1mol/��L?S��
��2����̼���ϲ�������3.36LΪ��������Ӧת�Ƶĵ��ӵ����ʵ���Ϊ
| 3.36L |
| 22.4L/mol |
�ʴ�Ϊ��1.806��1023
�ܸ���Hԭ���غ��֪���μӷ�ӦH+�����ʵ���Ϊ
| 3.36L |
| 22.4L/mol |
| 0.3mol |
| 0.2L |
�ʴ�Ϊ��1.5mol/L
��3��n��Na��=
| 2.3g |
| 23g/mol |
����Naԭ���غ�n��NaOD��=n��Na��=0.1mol������m��NaOD��=0.1mol��41g/mol=4.1g��
����ת�Ƶ����غ�n��D2��=
| 1 |
| 2 |
| 1 |
| 2 |
��Һ�����ʵ���������Ϊ
| 4.1g |
| 2.3g+mg-0.2g |
| 4.1 |
| 2.1+m |
�ʴ�Ϊ��
| 4.1 |
| 2.1+m |
���������黯ѧƽ�⼰��Ӧ���ʼ��㡢ԭ��ء���Һ���㣬�ۺϽϴ��ѶȽ�С����3������Һ������ȷ��Ϊ�״��㣬���������ɵ�D2�����װ�D2O��H2O���㣮

��ϰ��ϵ�д�
 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д� ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
�����Ŀ
��֪ij��Ӧ�ĸ�����Ũ���������£�aA��g��+bB��g��?cC��g��
��ʼŨ�ȣ�mol/L�� 3.0 1.0 0
2sĩŨ�ȣ�mol/L�� 1.8 0.6 0.8
�ɴ˿��Ƴ�����ʽ�и����ʵĻ�ѧ��������ϵ����֮��Ϊ��������
��ʼŨ�ȣ�mol/L�� 3.0 1.0 0
2sĩŨ�ȣ�mol/L�� 1.8 0.6 0.8
�ɴ˿��Ƴ�����ʽ�и����ʵĻ�ѧ��������ϵ����֮��Ϊ��������
| A��3��1��2 | B��2��1��3 | C��9��3��4 | D��3��2��1 |
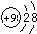
 2C��g��
2C��g�� +Br2��
+Br2��
 +HO-NO2
+HO-NO2 +H2O
+H2O
 2C��g��
2C��g��