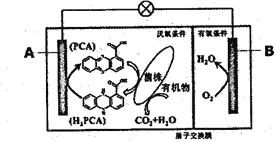
��Ŀ����
����Ŀ��X��Y��Z��W��R��M��N��7�ֶ�����Ԫ�أ�ԭ����������������֪X��ɵĵ������ܶ���С�����壬Y���γɻ�������������Ԫ�أ�Wԭ�������������Ǵ�����������3����R��M��N����ͬһ���ڣ�R�Ǹ������н�������ǿ��Ԫ�أ�R��N���γɻ�����RN��M��Wͬһ���塣
��ش��������⣺
��1��Z�����ڱ��е�λ����________________��N��ԭ�ӽṹʾ��ͼ��____________��
��2��X��Y�γɺ�̼����С���л�����ӵĿռ乹����_____________��
��3��X��W��N����ԭ�Ӹ�����1��1��1�γɵĻ�����ĵ���ʽΪ_____________��
��4��W��R�γɵĻ�����R2W2�������������û����ﺬ�еĻ�ѧ�������У� _________���û�������YW2��Ӧ�Ļ�ѧ����ʽ��__________________________________________________________��
��5����һ�������£���M���⻯���ˮ��Һ��ͨ��N�ĵ��ʣ����ֻ��ǣ���÷�Ӧ�Ļ�ѧ����ʽ��_________���˷�Ӧ��֤������������M___________N�����������������������
��6�����ϼ���Ԫ��������ϣ����γɵ�������ǿ��������________________________�����γɵļ�����ǿ��������_______________________����д��ѧʽ��
���𰸡��ڶ����ڵ�VA�� 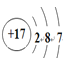 ��������
��������![]() ���Ӽ� ���ۼ���Ǽ��Թ��ۼ�2Na2O2��2CO2��2Na2CO3��O2Cl2��H2S��S����2HCl��HClO4NaOH
���Ӽ� ���ۼ���Ǽ��Թ��ۼ�2Na2O2��2CO2��2Na2CO3��O2Cl2��H2S��S����2HCl��HClO4NaOH
��������
X��ɵĵ������ܶ���С�����壬˵��XΪ��Ԫ�أ�Y���γɻ�������������Ԫ�أ�˵��Y��̼Ԫ�أ�Wԭ�������������Ǵ�����������3����˵��W����Ԫ�أ�R��M��N����ͬһ���ڣ�R�Ǹ������н�������ǿ��Ԫ�أ�˵��RΪ��Ԫ�أ�R��N���γɻ�����RN��˵��NΪ��Ԫ�أ�M��Wͬһ���壬˵��MΪ��Ԫ�أ�X��Y��Z��W��R��M��N��7�ֶ�����Ԫ�أ�ԭ��������������˵��ZΪ��Ԫ�أ��ݴ˷����ɵý��ۡ�
��1��ZΪ��Ԫ�أ�NΪ��Ԫ�أ��ʴ�Ϊ���ڶ����ڵ�VA�塢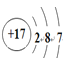 ��
��
��2��X��Y�γɺ�̼����С���л�������Ǽ�����ӣ���ռ乹��Ϊ�������壬�ʴ�Ϊ���������壻
��3��X��W��N����ԭ�Ӹ�����1��1��1�γɵĻ�����ΪHClO�������ʽΪ��![]() ����4��W��R�γɵĻ�����R2W2Ϊ�������ƣ��������Ӽ����ۼ����ʴ�Ϊ�����Ӽ������ۼ���Ǽ��Թ��ۼ��� 2Na2O2��2CO2��2Na2CO3��O2��
����4��W��R�γɵĻ�����R2W2Ϊ�������ƣ��������Ӽ����ۼ����ʴ�Ϊ�����Ӽ������ۼ���Ǽ��Թ��ۼ��� 2Na2O2��2CO2��2Na2CO3��O2��
��5��M���⻯���ˮ��Һ��ͨ��N�ĵ���Ϊ�û���Ӧ������ʵ˵���ȵķǽ�����ǿ���ʴ�Ϊ��Cl2��H2S��S����2HCl������
��6�����γɵ�������ǿ��������Ԫ�ص�����������Ӧ��ˮ�����HClO4�����γɵļ�����ǿ�����ʵ���Ԫ�ص�����������Ӧ��ˮ�����NaOH���ʴ�Ϊ��HClO4��NaOH��