��Ŀ����
����Ŀ��NiԪ�������������������Ź㷺��Ӧ�á��ش���������:
(1)��̬Niԭ�Ӽ۲���ӵ��Ų�ʽΪ_______��
(2)��ѧ�����о�������������ֵĹ����У�������Cu-Ni-Fe�ȶ��ֽ��������ȷ��ij�ֽ����������Ǿ��廹�ǷǾ�����ɿ��Ŀ�ѧ�����ǶԹ������______��
(3)Ni������±��(SCN)2��Ӧ����Ni(SCN)2��Ni(SCN)2�У���һ����������Ԫ����____��(SCN)2�����У���ԭ�ӵ��ӻ���ʽ��___��������������Ŀ֮��Ϊ_____��
(4)[Ni(NH3)6](NO3)2�У������ڵĻ�ѧ��Ϊ_____(����)��
a�����Ӽ� b�������� c����λ�� d�����
(5)����CO��N2���۷е�ߵ�ԭ��___��
(6)���Ͻ�����о���ȡ�úܴ��չ��
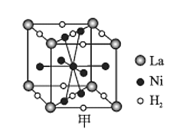
��ͼ����һ�������Ͻ����ľ����ṹʾ��ͼ���úϽ����1 mol La�ĺϽ������H2����ĿΪ___��
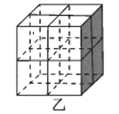
��Mg2NiH4��һ������Ľ����⻯���Mg2NiH4�����У�Niԭ��ռ����ͼ�ҵĶ�������ģ�Mg2+������ͼ�˸�С����������ġ�Mg2+λ��Niԭ���γɵ�___�������������϶�������������϶��������������ܶ�Ϊd g/cm3��Mg2NiH4��Ħ������ΪM g/mol����Mg2+��Niԭ�ӵ���̾���Ϊ___nm(�ú�d��M��NA�Ĵ���ʽ��ʾ)��
���𰸡�3d84s2 X-��������ʵ�� N sp3�ӻ� 5��4 b ����ͬ���ڷ��Ӿ��壬����Է���������ͬ��CO�ļ��Դ���N2�����Է��»��������۷е���� 3NA �������϶ 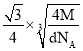 ��107
��107
��������
(1)Ni��28��Ԫ�أ����ݹ���ԭ���ɵ����������Ų�ʽ�������ɵ���۲���ӵ��Ų�ʽ��
(2)����X-��������ʵ��ȷ������Ĵ��ڣ�
(3)Ԫ�صķǽ�����Խǿ����縺�Ծ�Խ��(SCN)2���ӽṹ��ʽΪN��C-S-S-C��N������Sԭ�Ӽ۲���ӶԸ�����4�Һ���2���µ��Ӷԣ����ݼ۲���ӶԻ��������ж���ԭ�ӵ��ӻ���ʽ�����۵���Ϊ����������˫���к���һ��������һ�����������������к���1��������2��������
(4)����λ�������������ӻ�����������Ӽ������������к���λ�������ۼ���������ǻ�ѧ����
(5)���ݼ��Է��ӵķ��Ӽ��������ȷǼ��Է��ӵķ���֮���������������
(6)�ٸ��ݾ�����ԭ�ӷ�̯������La��Ni��H2��Ŀ��д����ѧʽ���ɽ��
��Niԭ��ռ�ݾ����Ķ�������ģ��������ֳ�8��С�����壬Mg2+������ͼ�˸�С����������ģ���λ��Niԭ���γɵ��������϶�ڣ�����Mg2+��Niԭ�ӵ���̾���Ϊ������Խ��ߵ��ķ�֮һ�������ܶȹ�ʽ���㾧���ı߳����ɽ��
(1)28��Ԫ��Ni�ĺ�������Ų�ʽ��1s22s22p63s23p63d84s2���ڲμӷ�Ӧʱ��Niԭ�ӵ�������4s���Ӻʹ�����3d���Ӷ����ܷ����仯�������۲���ӵ��Ų�ʽ��3d84s2��
(2)ȷ��ij�ֽ����������Ǿ��廹�ǷǾ�����ɿ��Ŀ�ѧ�����ǶԹ������X-��������ʵ�飻
(3)��Ni(SCN)2���漰����Ԫ����Ni��S��C��N����Ԫ�أ�����Ni�ǽ���Ԫ�أ��縺����С�������ַǽ���Ԫ��S��C��N�У�Ԫ�طǽ�������ǿ��Ԫ����N������NԪ�صĵ縺�����(SCN)2���ӽṹ��ʽΪN��C-S-S-C��N��Sԭ�Ӽ۲���ӶԸ�����4�Һ���2���µ��Ӷԣ����ݼ۲���ӶԻ������ۿ�֪��ԭ�ӵ��ӻ���ʽ��sp3�ӻ������ڹ��۵���Ϊ����������˫���к���һ��������һ�����������������к���1��������2����������(SCN)2�����к��е�������Ŀ��5����������Ŀ��4��������������������Ŀ֮��Ϊ5��4��
(4)[Ni(NH3)6](NO3)2�����ӻ����������[Ni(NH3)6]2+��NO3-ͨ�����Ӽ���ϣ���������[Ni(NH3)6]2+�У�����Ni2+��������λ��NH3֮��ͨ����λ����ϣ�����λ��NH3�У�N��H����Ԫ�ص�ԭ��֮��ͨ�����ۼ�N-H��ϣ������в�����������������ǻ�ѧ�������Բ����ڵĻ�ѧ��ѡ��Ϊb��
(5) CO��N2�����ڷ��Ӿ��壬����Է���������ͬ������CO�Ǽ��Է��ӣ�N2�ǷǼ��Է��ӣ�CO�ļ��Դ���N2�����Է��»����������CO�۷е��N2���ߣ�
(6)�پ����У�La����Ϊ8��![]() =1��Ni����Ϊ8��
=1��Ni����Ϊ8��![]() +1=5��H2����Ϊ2��
+1=5��H2����Ϊ2��![]() +8��
+8��![]() =3�������ʻ�ѧʽΪLaNi5(H2)3������1 mol La�ĺϽ������3 mol H2�����е�H2����ĿΪ3NA��
=3�������ʻ�ѧʽΪLaNi5(H2)3������1 mol La�ĺϽ������3 mol H2�����е�H2����ĿΪ3NA��
��Niԭ��ռ�ݾ����Ķ�������ģ��������ֳ�8��С�����壬Mg2+������ͼ�˸�С����������ģ���λ��Niԭ���γɵ��������϶�ڣ�����Mg2+��Niԭ�ӵ���̾���xΪ������Խ��ߵ��ķ�֮һ���辧���߳�Ϊa cm����x=![]() a cm����������8��Mg2+������������4����Mg2NiH4������������m=
a cm����������8��Mg2+������������4����Mg2NiH4������������m=![]() =da3g��a=
=da3g��a= cm����x=
cm����x=![]() a cm=
a cm=![]() ��
��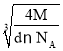 cm=
cm=![]() ��
��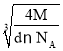 ��107nm��
��107nm��