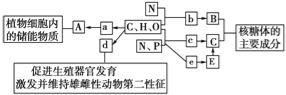
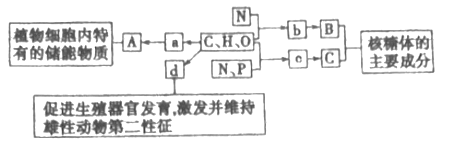
��Ŀ����
����Ŀ��0.3 molij��A����������ȫȼ�պ�����CO2��H2O��1.2 mol���Իش�
��1����A�ķ���ʽΪ_____________��
��2����ȡһ�����ĸ���A��ȫȼ�պ�����CO2��H2O��3mol������________g��A�μ��˷�Ӧ��ȼ��ʱ���ı�״���µ�����___________L��
��3������A��HCl��Ӧ�������ɲ����е�Hԭ�ӵĽṹ������ȫ��ͬ��д���÷�Ӧ�Ļ�ѧ����ʽ_____��
��4������A��Br2��CCl4��Һ��Ӧ��IJ���Ϊ1,2-���嶡�飬��д������A�����Ӿ۷�Ӧ�Ļ�ѧ����ʽ_________________________________________��
���𰸡� C4H8 42 100.8 ![]() + HCl��
+ HCl�� nCH2��CHCH2CH3
nCH2��CHCH2CH3![]()
![]()
����������1��0.3 molij��A����������ȫȼ�պ�����CO2��H2O��1.2 mol����1mol��������������ȫȼ�պ�����CO2��H2O��4 mol�����Է���ʽΪC4H8��
��2��ȡһ�����ĸ���A��C4H8����ȫȼ�պ�����CO2��H2O��3mol��������AΪ0.75mol������Ϊ0.75��56=42g����ӦΪC4H8 + 6O2 = 4CO2 + 4H2O������0.75mol C4H8��Ҫ������Ϊ0.75��6��22.4=100.8L��
��3����A��HCl��Ӧ���÷�Ӧֻ���Ǽӳɣ�˵��AΪ��ϩ���ӳɺ�һ���õ�һ�ȴ����飬Hԭ�ӵĽṹ������ȫ��ͬ�����һ�ȴ�����ֻ���� ������AΪ
������AΪ![]() ����ӦΪ
����ӦΪ![]() + HCl��
+ HCl�� ��
��
��4����A��Br2��CCl4��Һ��Ӧ��IJ���Ϊ1,2-���嶡�飬��AΪ1-��ϩ��A�����Ӿ۷�Ӧ�Ļ�ѧ����ʽΪnCH2��CHCH2CH3![]()
![]()