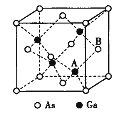
ЬтФПФкШн
ЁОЬтФПЁПбЧЬњЧшЛЏМиЃЈK4[Fe(CN)6])ЫЋГЦЛЦбЊбЮЃЌЪЧвЛжжживЊЕФЛЏЙЄдСЯЁЃМьбщШ§МлЬњЗЂЩњЕФЗДгІЮЊЃКK4[Fe(CN)6]+FeCl3=KFe[Fe(CN)6]Ё§(ыјЪЯРЖ)+3KClЃЌЛиД№ЮЪЬтЃК
ЃЈ1ЃЉаДГіЛљЬЌFe3+ЕФКЫЭтЕчзгХХВМЪН___ЁЃ
ЃЈ2ЃЉK4[Fe(CN)6]жаЕФзїгУСІГ§ЙВМлМќЭтЃЌЛЙга___КЭ___ЁЃ
ЃЈ3ЃЉЛЦбЊбЮжаNдзгЕФдгЛЏЗНЪНЮЊ____ЃЛCЁЂNЁЂOЕФЕквЛЕчРыФмгЩДѓЕНаЁЕФХХађЮЊ___ЃЌЕчИКадгЩДѓЕНаЁЕФХХађЮЊ___ЁЃ
ЁОД№АИЁП1s22s22p63s23p63d5Лђ[Ar]3d5 ХфЮЛМќ РызгМќ sp N>O>C O>N>C
ЁОНтЮіЁП
(1)FeдзгКЫЭтга26ИіЕчзгЃЌЪЇШЅЭтЮЇ3ИіЕчзгаЮГЩFe3+ЃЌЫљвдЛљЬЌFe3+ЕФКЫЭтЕчзгХХВМЪНЮЊ1s22s22p63s23p63d5Лђ[Ar]3d5ЃЛ
(2)K4[Fe(CN)6]ЪЧРызгЛЏКЯЮяЃЌДцдкРызгМќЃЌЦфжаFe(CN)64-КЌгаЙВМлМќКЭХфЮЛМќЃЌМДЛЏбЇМќЮЊРызгМќЁЂХфЮЛМќКЭЙВМлМќЃЛ
(3)CN-жаNЕФМлВуЕчзгЖдЪ§=1+![]() =2ЃЌЫљвдNВЩгУspдгЛЏЃЛЭЌвЛжмЦкдЊЫиЕквЛЕчРыФмЫцзХдзгађЪ§діДѓЖјГЪдіДѓЧїЪЦЃЌЕЋЪЧЕкIIAзхЁЂЕкVAзхдЊЫиЕквЛЕчРыФмДѓгкЦфЯрСкдЊЫиЃЌЫљвдCЁЂNЁЂOЕквЛЕчРыФмДѓаЁЫГађЮЊNЃОOЃОCЃЛЭЌвЛжмЦкдЊЫиЕчИКадЫцзХдзгађЪ§діДѓЖјдіДѓЃЌЫљвдCЁЂNЁЂOЕФЕчИКадДѓаЁЫГађЮЊOЃОNЃОCЁЃ
=2ЃЌЫљвдNВЩгУspдгЛЏЃЛЭЌвЛжмЦкдЊЫиЕквЛЕчРыФмЫцзХдзгађЪ§діДѓЖјГЪдіДѓЧїЪЦЃЌЕЋЪЧЕкIIAзхЁЂЕкVAзхдЊЫиЕквЛЕчРыФмДѓгкЦфЯрСкдЊЫиЃЌЫљвдCЁЂNЁЂOЕквЛЕчРыФмДѓаЁЫГађЮЊNЃОOЃОCЃЛЭЌвЛжмЦкдЊЫиЕчИКадЫцзХдзгађЪ§діДѓЖјдіДѓЃЌЫљвдCЁЂNЁЂOЕФЕчИКадДѓаЁЫГађЮЊOЃОNЃОCЁЃ

ЁОЬтФПЁПЂёЁЂбаОПадбЇЯАаЁзщНјааSO2ЕФжЦБИМАаджЪЬНОПЪЕбщЃЌзАжУШчЭМЃЈaЮЊЛюШћЃЌМгШШМАЙЬЖЈзАжУвбТдШЅЃЉЁЃ
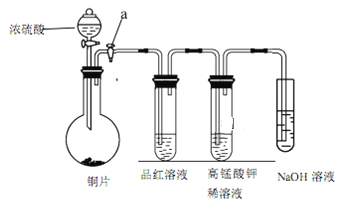
ЃЈ1ЃЉСЌНгвЧЦїЁЂ___ЁЂМгвЉЦЗКѓЃЌДђПЊ aЃЌШЛКѓЕЮШыХЈСђЫсЃЌМгШШЃЛ
ЃЈ2ЃЉЭгыХЈСђЫсЗДгІжЦБИ SO2ЕФЛЏбЇЗНГЬЪНЪЧ___ЃЛ
ЃЈ3ЃЉЦЗКьШмвКжаЕФЪЕбщЯжЯѓЪЧ___ЃЛ
ЃЈ4ЃЉДгИпУЬЫсМиШмвКжаЙлВьЕНЕФЯжЯѓЫЕУї SO2Опга___адЁЃ
ЂђЁЂЩЯЪіЪЕбщжа NaOH ШмвКгУгкЮќЪеЪЃгрЕФ SO2 ЩњГЩ Na2SO3ЃЌNa2SO3ЪЧПЙбѕМСЁЃЯђЩеМюКЭNa2SO3ЛьКЯШмвКжаМгШыЩйаэфхЫЎЃЌеёЕДКѓШмвКБфЮЊЮоЩЋЁЃ
ЃЈ1ЃЉаДГідкМюадШмвКжаBr2бѕЛЏNa2SO3ЕФРызгЗНГЬЪН___
ЃЈ2ЃЉЗДгІКѓЕФШмвККЌгаSO32ЃЁЂSO42ЃЁЂBrЃЁЂOHЃЕШвѕРызгЃЌЧыЬюаДМјЖЈЦфжаSO42ЃКЭBrЃЕФЪЕбщБЈИцЁЃ___
ЯобЁЪдМСЃК2molЁЄLЃ1HClЃЛ1molЁЄLЃ1H2SO4ЃЛlmolЁЄLЃ1BaCl2ЃЛlmolЁЄLЃ1BaЃЈNO3ЃЉ2ЃЛ0.1molЁЄLЃ1AgNO3ЃЛCCl4ЃЛаТжЦТШЫЎЁЃ
БрКХ | ЪЕбщВйзї | дЄЦкЯжЯѓКЭНсТл |
ВНжшЂй | ШЁЩйСПД§ВтвКМгШыЪдЙмжаЃЌМгШыЙ§СПЕФ2molЁЄLЃ1бЮЫсЃЌдйЕЮМг | га ЩњГЩЃЌжЄУїД§ВтвКжаSO42- |
ВНжшЂк | ШЁГіВНжшЂйжаЪЪСПЩЯВуЧхвКгкЪдЙмжаЃЌМгШыЪЪСПТШЫЎЃЌдйМгШы ЃЌеёЕДЃЌОВжУЁЃ | ЯТВувКЬхГЪ ЃЌжЄУїД§ВтвКжаКЌBr-ЁЃ |
ЁОЬтФПЁПРћгУЛЏбЇдРэПЩвдЖдЙЄГЇХХЗХЕФЗЯЫЎЁЂЗЯдќЕШНјаагааЇМьВтгыКЯРэДІРэЁЃФГЙЄГЇЖджЦИяЙЄвЕЮлФржаCr(Ђѓ)ЛиЪегыдйРћгУЙЄвеШчЯТЃЈСђЫсНўШЁвКжаН№ЪєРызгжївЊЪЧCr3+ЃЌЦфДЮЪЧFe3+ЁЂAl3+ЁЂCa2+ЁЂMg2+ЃЉЃК

ВПЗжбєРызгГЃЮТЯТвдЧтбѕЛЏЮяаЮЪНГСЕэЪБШмвКЕФpHМћЯТБэЃК
бєРызг | Fe3+ | Fe2+ | Mg2+ | Al3+ | Cu2+ | Cr3+ |
ПЊЪМГСЕэЪБЕФpH | 1.9 | 7.0 | ЁЊЁЊ | ЁЊЁЊ | 4.7 | ЁЊЁЊ |
ГСЕэЭъШЋЪБЕФpH | 3.2 | 9.0 | 11.1 | 8 | 6.7 | 9ЃЈЃО9ШмНтЃЉ |
ЃЈ1ЃЉЪЕбщЪвгУ18.4 molЁЄL-1ЕФХЈСђЫсХфжЦ250 mL 4.8 molЁЄL-1ЕФСђЫсШмвКЃЌЫљгУЕФВЃСЇвЧЦїГ§ЩеБЁЂВЃСЇАєКЭЮќСПЙмЃЈвЛжжФмОЋШЗСПШЁвЛЖЈЬхЛ§вКЬхЕФвЧЦїЃЉЭтЃЌЛЙаш______________________ЁЃ
ЃЈ2ЃЉЫсНўЪБЃЌЮЊСЫЬсИпНўШЁТЪПЩВЩШЁЕФДыЪЉга___________________________ЁЃ(аДГіСНИіДыЪЉ)
ЃЈ3ЃЉМгШыH2O2ЕФзїгУЪЧ_____________________________________ЁЃ
ЃЈ4ЃЉМгШыNaOHШмвКЪЙШмвКГЪМюадЃЌCr2O72ЈDзЊЛЏЮЊCrO42ЈDЁЃТЫвКЂђжабєРызгжївЊга______ЃЛЕЋШмвКЕФpHВЛФмГЌЙ§8ЃЌЦфРэгЩЪЧ_______________ЁЃ
ЃЈ5ЃЉФЦРызгНЛЛЛЪїжЌЕФЗДгІдРэЮЊЃКMn+ЃЋnNaRЁњMRnЃЋnNa+ЃЌРћгУФЦРызгНЛЛЛЪїжЌГ§ШЅТЫвКЂђжаЕФН№ЪєбєРызгЪЧ___________________ЁЃ
ЃЈ6ЃЉаДГіЩЯЪіСїГЬжагУSO2НјааЛЙдЕФРызгЗНГЬЪН___________________________ЁЃ