��Ŀ����
��9�֣����з�Ӧ��mA(g) + nB(g)  pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C������������С����
pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C������������С����
(1) �÷�Ӧ������ӦΪ ________��Ӧ�������Ȼ���ȣ�����S_____0 (���������=������)���ɴ��жϣ��˷�Ӧ�Է����е�����Ϊ_______��
A һ���Է� Bһ�����Է� C�����Է������²��Է� D�����Է������²��Է�
(2) ��ѹʱ��A����������_________��(���������С�����䡱����ͬ)
(3) ������B (�������)����A��ת����_________��
(4) �������¶ȣ���ƽ��ʱB��C��Ũ��֮��C(B) ��C(C) ��_________��
(5) �����������ƽ��ʱ��������������ʵ���____ ___��
(6) ��B����ɫ���ʣ�A��C����ɫ�������C (�������)��������ɫ_______��(����������dz�����䡱����ͬ)
��7��ά��������ѹǿ���䣬���������������ɫ ��
 pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C������������С����
pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C������������С����(1) �÷�Ӧ������ӦΪ ________��Ӧ�������Ȼ���ȣ�����S_____0 (���������=������)���ɴ��жϣ��˷�Ӧ�Է����е�����Ϊ_______��
A һ���Է� Bһ�����Է� C�����Է������²��Է� D�����Է������²��Է�
(2) ��ѹʱ��A����������_________��(���������С�����䡱����ͬ)
(3) ������B (�������)����A��ת����_________��
(4) �������¶ȣ���ƽ��ʱB��C��Ũ��֮��C(B) ��C(C) ��_________��
(5) �����������ƽ��ʱ��������������ʵ���____ ___��
(6) ��B����ɫ���ʣ�A��C����ɫ�������C (�������)��������ɫ_______��(����������dz�����䡱����ͬ)
��7��ά��������ѹǿ���䣬���������������ɫ ��
��ÿ��һ�֣�
��1������ �� B ��2������3������4����С ��5������ ��6�����7����dz
��1������ �� B ��2������3������4����С ��5������ ��6�����7����dz
��1�����淴ӦmA(g) + nB(g)  pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ��˵��ƽ������Ӧ�����ȷ�Ӧ����H>0������Сѹǿʱ�������ϵ��C������������С��˵��ƽ�����ƣ�����Ӧ������ϵ����С�ķ�Ӧ������S<0���ɡ�G����H��T��S��֪����G>0����һ�����Է����С�
pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ��˵��ƽ������Ӧ�����ȷ�Ӧ����H>0������Сѹǿʱ�������ϵ��C������������С��˵��ƽ�����ƣ�����Ӧ������ϵ����С�ķ�Ӧ������S<0���ɡ�G����H��T��S��֪����G>0����һ�����Է����С�
��2����ѹʱ��ƽ�����ƣ�A��������������
��3��������B (�������)��ƽ�����ƣ���A��ת��������
(4) �������¶ȣ���ƽ�����ƣ�ƽ��ʱʱB��C��Ũ��֮��C(B) ��C(C) ����С
(5) �����������ƽ�ⲻ���ƶ���������Ũ�Ȳ��䣬ƽ��ʱ��������������ʵ�������
(6) ��B����ɫ���ʣ�A��C����ɫ�������C (�������)��ƽ�����ƣ�B��Ũ�����������ɫ����
��7��ά��������ѹǿ���䣬��������������Ũ�Ⱦ���Լ�С���������ɫһ����dz��
 pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ��˵��ƽ������Ӧ�����ȷ�Ӧ����H>0������Сѹǿʱ�������ϵ��C������������С��˵��ƽ�����ƣ�����Ӧ������ϵ����С�ķ�Ӧ������S<0���ɡ�G����H��T��S��֪����G>0����һ�����Է����С�
pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ��˵��ƽ������Ӧ�����ȷ�Ӧ����H>0������Сѹǿʱ�������ϵ��C������������С��˵��ƽ�����ƣ�����Ӧ������ϵ����С�ķ�Ӧ������S<0���ɡ�G����H��T��S��֪����G>0����һ�����Է����С���2����ѹʱ��ƽ�����ƣ�A��������������
��3��������B (�������)��ƽ�����ƣ���A��ת��������
(4) �������¶ȣ���ƽ�����ƣ�ƽ��ʱʱB��C��Ũ��֮��C(B) ��C(C) ����С
(5) �����������ƽ�ⲻ���ƶ���������Ũ�Ȳ��䣬ƽ��ʱ��������������ʵ�������
(6) ��B����ɫ���ʣ�A��C����ɫ�������C (�������)��ƽ�����ƣ�B��Ũ�����������ɫ����
��7��ά��������ѹǿ���䣬��������������Ũ�Ⱦ���Լ�С���������ɫһ����dz��

��ϰ��ϵ�д�
 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д�
�����Ŀ

 2NH3(g)��CO2(g)��ʵ���ò�ͬ�¶��µ�ƽ�����������±���
2NH3(g)��CO2(g)��ʵ���ò�ͬ�¶��µ�ƽ�����������±���
 2NH3��5 min��ﵽƽ�⣬�úϳɷ�Ӧ5 min�ڵ�����v(NH3)=" 0.02" mol/��L��min��������ƽ��ʱ��������N2ת������ ��
2NH3��5 min��ﵽƽ�⣬�úϳɷ�Ӧ5 min�ڵ�����v(NH3)=" 0.02" mol/��L��min��������ƽ��ʱ��������N2ת������ �� S��g����R��g�����ں��º����ܱ������еķ�Ӧ�Ѵ�ƽ�����
S��g����R��g�����ں��º����ܱ������еķ�Ӧ�Ѵ�ƽ�����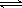 2NH3(g) ��
2NH3(g) �� NO2(g)
NO2(g) 2N2(g)
2N2(g)
 C(g)+D(g) �ں������Ѵﵽƽ����ǣ� ��
C(g)+D(g) �ں������Ѵﵽƽ����ǣ� ��