��Ŀ����
��15�֣�X��Y��ZΪ���ڱ���ǰ20������Ԫ�أ�ԭ������������X��Yԭ�ӵ����������� ������Ӳ�����2����Z�������к�����ߵĽ���Ԫ�ء�
������Ӳ�����2����Z�������к�����ߵĽ���Ԫ�ء�
��1�����к��������ѧʽ��д����ȷ���� ������ţ���
a��XO3- b��XO32- c��YO32- d��Y2O32-
��2��X��Y��ԭ�ӿɹ���ֻ�����Լ��ķǼ��Է��ӣ����ĵ���ʽ�� ���ռ乹���� ��
��3��Y����ۺ���������Ҫ�Ļ�����Ʒ��
����֪YO2������������ÿ����1mol��̬YO3���ų�98��3kJ�������÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��ʵ������ͬ������һ������Y���ʷֱ��ڿ������������г��ȼ�պ����ijɷ֣���������������Y�ڴ�����ȼ�յIJ�����YO3�����ȿ������٣��Է�����ԭ�� ��
 ������Ӳ�����2����Z�������к�����ߵĽ���Ԫ�ء�
������Ӳ�����2����Z�������к�����ߵĽ���Ԫ�ء���1�����к��������ѧʽ��д����ȷ���� ������ţ���
a��XO3- b��XO32- c��YO32- d��Y2O32-

��2��X��Y��ԭ�ӿɹ���ֻ�����Լ��ķǼ��Է��ӣ����ĵ���ʽ�� ���ռ乹���� ��
��3��Y����ۺ���������Ҫ�Ļ�����Ʒ��
����֪YO2������������ÿ����1mol��̬YO3���ų�98��3kJ�������÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��ʵ������ͬ������һ������Y���ʷֱ��ڿ������������г��ȼ�պ����ijɷ֣���������������Y�ڴ�����ȼ�յIJ�����YO3�����ȿ������٣��Է�����ԭ�� ��
| | YO2 | YO3 |
| ���� | 94%��95% | 5%��6% |
| ���� | 97%��98% | 2%��3% |
��1��a��3�֣���2��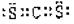 ��3�֣���ֱ���ͣ�3�֣�
��3�֣���ֱ���ͣ�3�֣�
��3����SO2��g��+1/2O2��g��==SO3��g������H=��98.3kJ��mol-1��3�֣�
�ڴ�����O2��Ũ�ȴ�λʱ���ڷ��ȶ࣬��ϵ�¶ȸߣ�ƽ����SO3�ֽ�ķ����ƶ���
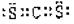 ��3�֣���ֱ���ͣ�3�֣�
��3�֣���ֱ���ͣ�3�֣���3����SO2��g��+1/2O2��g��==SO3��g������H=��98.3kJ��mol-1��3�֣�
�ڴ�����O2��Ũ�ȴ�λʱ���ڷ��ȶ࣬��ϵ�¶ȸߣ�ƽ����SO3�ֽ�ķ����ƶ���
��

��ϰ��ϵ�д�
�����Ŀ


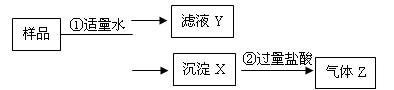

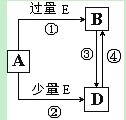
 ____��
____��