��Ŀ����
����Ŀ��A��B��C��D��E��Ϊ�л������A�ǻ�ѧʵ���г������л����������ˮ����������ζ��B�IJ����ɺ���һ������ʯ�ͻ�����չ��ˮƽ���й����ʵ�ת����ϵ��ͼ����ʾ��
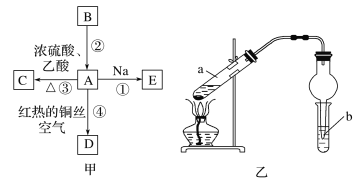
��1��д��B�Ľṹ��ʽ__��A�й����ŵ�����Ϊ__��
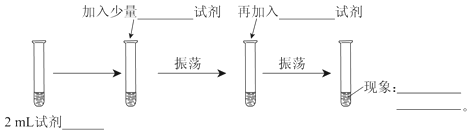
��2����֤�л��������к�����COOH����Լ���__��ʵ��������__��
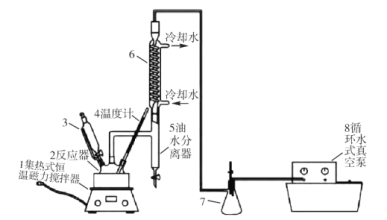
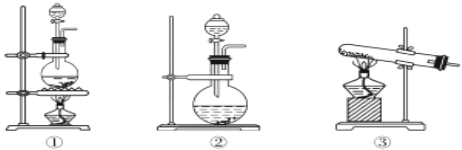
��3��ʵ�������÷�Ӧ����ȡC������ͼ��װ�ã�
��a�Թ�����Ҫ��Ӧ�Ļ�ѧ����ʽΪ___����Ӧ������___��
����ʵ�������θ���ܳ������������⣬��һ����Ҫ������__��
���Թ�b�й۲쵽��������___��
���𰸡�CH2=CH2 �ǻ� ʯ�� ��� CH3CH2OH+CH3COOH![]() CH3COOCH2CH3+H2O ������ȡ����Ӧ ��ֹ���� �ֲ㣨����ζ��
CH3COOCH2CH3+H2O ������ȡ����Ӧ ��ֹ���� �ֲ㣨����ζ��
��������
B�IJ����ɺ���һ������ʯ�ͻ�����չ��ˮƽ��BΪCH2�TCH2��A�ǻ�ѧʵ���г������л����������ˮ����������ζ�����ͼ��ת����֪��AΪCH3CH2OH��CH3CH2OH��Na��Ӧ����EΪCH3CH2ONa��CH3CH2OH�����ᷴӦ����CΪCH3COOCH2CH3��A������������Ӧ����DΪCH3CHO��
������������֪��
��1��BΪ��ϩ�����Ľṹ��ʽΪCH2�TCH2��AΪ�Ҵ����ṹ��ʽΪCH3CH2OH��A�й���������Ϊ�ǻ�����ΪCH2�TCH2���ǻ���
��2�������к�����COOH�������ԣ���ʯ���Լ����ɼ��飬��ʯ���Լ���죬��֤�������к�����COOH����Ϊʯ���졣
��3�����Ҵ���������ŨH2SO4���ȵ������·�����ѧ��Ӧ����������������ˮ����ѧ����ʽΪ��CH3CH2OH+CH3COOH![]() CH3COOCH2CH3+H2O����Ӧ����Ϊ������ȡ������Ӧ����ΪCH3CH2OH+CH3COOH
CH3COOCH2CH3+H2O����Ӧ����Ϊ������ȡ������Ӧ����ΪCH3CH2OH+CH3COOH![]() CH3COOCH2CH3+H2O��������ȡ������Ӧ��
CH3COOCH2CH3+H2O��������ȡ������Ӧ��
����ʵ�������θ���ܳ������������⣬��һ����Ҫ�����Ƿ�ֹ��������Ϊ��ֹ������
������������������к������ᡢ�Ҵ���ͨ���ñ���̼������Һ����ȥ�ӷ�������������Ҵ�����������������ˮ���ܶȱ�ˮС��Һ��ֲ㣨����ζ������ΪҺ��ֲ㣨����ζ����

 ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д�