ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ“―÷ΣΘΚCO2(g)ΘΪ3H2(g)![]() CH3OH(g)ΘΪH2O(g) ΠΛHΘΫΘ≠49.0kJΓΛmolΘ≠1ΓΘ“ΜΕ®ΧθΦΰœ¬Θ§œρΧεΜΐΈΣ1LΒΡΚψ»ίΟή±’»ίΤς÷–≥δ»κ1molCO2ΚΆ3mol H2Θ§≤βΒΟCO2ΚΆCH3OH(g)ΒΡ≈®Ε»Υφ ±Φδ±δΜ·«ζœΏ»γΆΦΥυ ΨΓΘœ¬Ν––π ω÷–’ΐ»ΖΒΡ «Θ® Θ©
CH3OH(g)ΘΪH2O(g) ΠΛHΘΫΘ≠49.0kJΓΛmolΘ≠1ΓΘ“ΜΕ®ΧθΦΰœ¬Θ§œρΧεΜΐΈΣ1LΒΡΚψ»ίΟή±’»ίΤς÷–≥δ»κ1molCO2ΚΆ3mol H2Θ§≤βΒΟCO2ΚΆCH3OH(g)ΒΡ≈®Ε»Υφ ±Φδ±δΜ·«ζœΏ»γΆΦΥυ ΨΓΘœ¬Ν––π ω÷–’ΐ»ΖΒΡ «Θ® Θ©
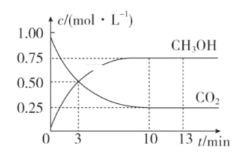
A.3min ±Θ§”ΟCO2ΒΡ≈®Ε»±μ ΨΒΡΠ‘(’ΐ)Β»”Ύ”ΟCH3OHΒΡ≈®Ε»±μ ΨΒΡΠ‘(Ρφ)
B.¥”0-10minΘ§”ΟH2±μ ΨΒΡΤΫΨυΖ¥”ΠΥΌ¬ v(H2)ΘΫ0.75molΓΛLΘ≠1ΓΛminΘ≠1
C.13min ±Θ§œρ»ίΤς÷–≥δ»κ2molΚΛΤχΘ§ΗΟΖ¥”ΠΒΡΜ·―ßΖ¥”ΠΥΌ¬ ‘ωΦ”
D.¥”Ζ¥”Π¥οΒΫΤΫΚβΉ¥Χ§ ±Θ§CO2ΒΡΤΫΚβΉΣΜ·¬ ΈΣ75%
ΓΨ¥πΑΗΓΩD
ΓΨΫβΈωΓΩ
A. ”…ΆΦœσΩ…÷ΣΘ§‘Ύ3min ±Θ§c(CO2)=c(CH3OH)Θ§ΒΪΖ¥”ΠΟΜ”–¥οΒΫΤΫΚβΉ¥Χ§Θ§v(’ΐ)ΓΌv(Ρφ)Θ§Aœν¥μΈσΘΜ
B. v(H2)ΘΫ![]() =
=![]() =0.225 molΓΛLΘ≠1ΓΛminΘ≠1Θ§Bœν¥μΈσΘΜ
=0.225 molΓΛLΘ≠1ΓΛminΘ≠1Θ§Bœν¥μΈσΘΜ
C. 13min ±Θ§œρ»ίΤς÷–≥δ»κ2molΚΛΤχΚσΘ§»ίΤςΒΡΒΡ»ίΜΐ≤Μ±δΘ§ΗςΈο÷ ΒΡ≈®Ε»≤Μ±δΘ§Ζ¥”ΠΥΌ¬ ≤Μ±δΘ§Cœν¥μΈσΘΜ
D. Ζ¥”Π¥οΒΫΤΫΚβΉ¥Χ§ ±Θ§CO2ΒΡΤΫΚβΉΣΜ·¬ =![]() ΓΝ100%=75%Θ§Dœν’ΐ»ΖΘΜ
ΓΝ100%=75%Θ§Dœν’ΐ»ΖΘΜ
¥πΑΗ―ΓDΓΘ

 ‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗ
‘ΡΕΝΩλ≥ΒœΒΝ–¥πΑΗΓΨΧβΡΩΓΩ“―÷Σ25Γφ ±”–ΙΊ»θΥαΒΡΒγάκΤΫΚβ≥Θ ΐΘΚ‘ρœ¬Ν–”–ΙΊΥΒΖ®’ΐ»ΖΒΡ «Θ® Θ©
»θΥαΜ·―ß Ϋ | CH3COOH | HCN | H2CO3 |
ΒγάκΤΫΚβ≥Θ ΐ | 1.8ΓΝ10-5 | 4.9ΓΝ10-10 | K1ΘΫ4.3ΓΝ10-7 K2ΘΫ5.6ΓΝ10-11 |
A. Β»Έο÷ ΒΡΝΩ≈®Ε»ΒΡΗς»ή“ΚpHΙΊœΒΈΣΘΚpH(NaCN)ΘΨpH(Na2CO3)ΘΨpH(CH3COONa)
B. ±υ¥ΉΥα÷–÷πΒΈΦ”Υ°Θ§‘ρ»ή“ΚΒΡΒΦΒγ–‘ΓΔ¥ΉΥαΒΡΒγάκ≥ΧΕ»ΓΔpHΨυœ»‘ω¥σΚσΦθ–Γ
C. NaCN÷–Ά®»κ…ΌΝΩCO2ΖΔ…ζΒΡΜ·―ßΖ¥”ΠΈΣΘΚNaCN+CO2+H2O=HCN+NaHCO3
D. œΓ ΆHCN»ή“ΚΙΐ≥Χ÷–Θ§![]() Φθ–Γ
Φθ–Γ
ΓΨΧβΡΩΓΩ800Γφ ±Θ§‘Ύ2LΒΡΟή±’»ίΤς÷–ΖΔ…ζΖ¥”ΠΘΚ2NO(g)+O2(g)![]() 2NO2Θ§n(NO)Υφ ±ΦδΒΡ±δΜ·»γœ¬±μΥυ ΨΓΘ
2NO2Θ§n(NO)Υφ ±ΦδΒΡ±δΜ·»γœ¬±μΥυ ΨΓΘ
±Φδ®Ms | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO)®Mmol | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
Θ®1Θ©¥”0ΓΪ3sΡΎΘ§”ΟNO±μ ΨΒΡΤΫΨυΖ¥”ΠΥΌ¬ Π‘(NO)ΘΫ_____________ΓΘ
Θ®2Θ©ΆΦ÷–±μ ΨNO≈®Ε»±δΜ·ΒΡ«ζœΏ «_______(ΧνΉ÷ΡΗ¥ζΚ≈)ΓΘ
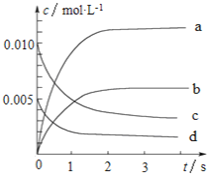
Θ®3Θ©¥οΤΫΚβ ±NOΒΡΉΣΜ·¬ ΈΣ________ΓΘ
Θ®4Θ©ΡήΥΒΟςΗΟΖ¥”Π“―¥οΒΫΤΫΚβΉ¥Χ§ΒΡ «_________(Χν–ρΚ≈)ΓΘ
AΘ°ΜλΚœΤχΧεΒΡ―’…Ϊ±Θ≥÷≤Μ±δ
BΘ°ΜλΚœΤχΧεΒΡΟήΕ»±Θ≥÷≤Μ±δ
CΘ°Π‘Ρφ (NO2)ΘΫ2Π‘’ΐ (O2)
DΘ°ΜλΚœΤχΧεΒΡΤΫΨυœύΕ‘Ζ÷Ή”÷ ΝΩ±Θ≥÷≤Μ±δ
Θ®5Θ©»τ…ΐΈ¬ΒΫ850ΓφΘ§¥οΤΫΚβΚσn(NO)ΘΫn(NO2)Θ§‘ρΖ¥”Πœρ________(ΧνΓΑ’ΐΖ¥”ΠΖΫœρΓ±ΓΔΓΑΡφΖ¥”ΠΖΫœρΓ±)“ΤΕ·ΓΘ
Θ®6Θ©»τ‘Ύ“ΜΕ®ΧθΦΰœ¬0.2molNOΤχΧε”κ―θΤχΖ¥”ΠΘ§¥οΤΫΚβ ±≤βΒΟΖ≈≥ωΒΡ»»ΝΩΈΣakJΘ§¥Υ ±NOΉΣΜ·¬ ΈΣ80%Θ§‘ρ2molNOΤχΧεΆξ»ΪΖ¥”ΠΖ≈≥ωΒΡ»»ΝΩΈΣ____________ΓΘ
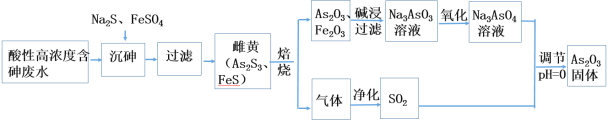
ΓΨΧβΡΩΓΩ¥ΩΙΐ―θΜ·«β «Β≠άΕ…ΪΒΡπΛ≥μ“ΚΧεΘ§Ω…”κΥ°“‘»Έ“β±»ΜλΚœΘ§ΤδΥ°»ή“ΚΥΉ≥ΤΥΪ―θΥ°Θ§ΈΣΈό…ΪΆΗΟς“ΚΧεΓΘ Β―ι “≥Θ”ΟΙΐ―θΜ·«β÷Τ»Γ―θΤχΘ§ΙΛ“Β…œΙΐ―θΜ·«β «÷Ί“ΣΒΡ―θΜ·ΦΝΚΆΜΙ‘≠ΦΝΘ§≥Θ”Ο”ΎœϊΕΨΓΔ…±ΨζΓΔΤ·ΑΉΒ»ΓΘΡ≥Μ·―ß–Υ»Λ–ΓΉιΒΡΆ§―ßΈß»ΤΙΐ―θΜ·«βΩΣ’ΙΝΥΒς≤ι―–ΨΩ”κ Β―ιΘ§«κΡψ≤Έ”κΤδ÷–“ΜΤπΆξ≥…œ¬Ν–―ßœΑ»ΈΈώΘΚ
Θ®1Θ©–¥≥ωΙΐ―θΜ·«βΒΡΒγΉ” ΫΘΚ_____ΓΘ
Θ®2Θ© Β―ι “÷–”ΟΙΐ―θΜ·«β÷Τ»Γ―θΤχΒΡΜ·―ßΖΫ≥Χ ΫΈΣ____Θ§Β±…ζ≥…±ξΉΦΉ¥Ωωœ¬1.12 L O2 ±Θ§ΉΣ“ΤΒγΉ”ΈΣ___ molΓΘ
Θ®3Θ©ΗΟ–Υ»Λ–ΓΉιΒΡΆ§―ß≤ι‘ΡΉ ΝœΚσΖΔœ÷H2O2ΈΣΕΰ‘Σ»θΥαΘ§ΤδΥα–‘±»ΧΦΥα»θΓΘ«κ–¥≥ωH2O2‘ΎΥ°»ή“Κ÷–ΒΡΒγάκΖΫ≥Χ ΫΘΚ___________ΓΘ
Θ®4Θ©Ά§―ßΟ«”Ο0.1000 molΓΛLΘ≠1ΒΡΥα–‘ΗΏΟΧΥαΦΊ±ξΉΦ»ή“ΚΒΈΕ®Ρ≥ ‘―υ÷–Ιΐ―θΜ·«βΒΡΚ§ΝΩΓΘ
ΔΌ–¥≥ωΗΟΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ Ϋ____________ΓΘ
ΔΎΒΈΕ®ΒΫ¥ο÷’ΒψΒΡœ÷œσ «____________ΓΘ
Δέ”Ο“Τ“ΚΙήΈϋ»Γ25.00 mL ‘―υ÷Ο”ΎΉΕ–ΈΤΩ÷–Θ§÷ΊΗ¥ΒΈΕ®ΥΡ¥ΈΘ§ΟΩ¥ΈœϊΚΡΒΡΥα–‘KMnO4±ξΉΦ»ή“ΚΧεΜΐ»γœ¬±μΥυ ΨΘΚ
ΒΎ“Μ¥Έ | ΒΎΕΰ¥Έ | ΒΎ»ΐ¥Έ | ΒΎΥΡ¥Έ | |
ΧεΜΐ(mL) | 17.10 | 19.10 | 17.00 | 16.90 |
‘ρ ‘―υ÷–Ιΐ―θΜ·«βΒΡ≈®Ε»ΈΣ____molΓΛLΘ≠1ΓΘ
Δή»τΒΈΕ®«ΑΦβΉλ÷–”–Τχ≈ίΘ§ΒΈΕ®Κσœϊ ßΘ§‘ρ≤βΕ®ΫαΙϊ____(ΧνΓΑΤΪΒΆΓ±ΓΔΓΑΤΪΗΏΓ±ΜρΓΑ≤Μ±δΓ±)ΓΘ
Θ®5Θ©Ά§―ßΟ«ΖΔœ÷œρΒΈΦ”ΝΥΖ”ΧΣΒΡNaOH»ή“Κ÷–Φ”»κH2O2ΚσΘ§»ή“Κ÷–Κλ…Ϊœϊ ßΓΘΙΊ”ΎΆ …Ϊ‘≠“ρΘΚΦΉΆ§―ß»œΈΣH2O2 «Εΰ‘Σ»θΥαΘ§œϊΚΡΝΥOHΘ≠ ΙΚλ…Ϊœϊ ßΘΜ““Ά§―ß»œΈΣH2O2ΨΏ”–Τ·ΑΉ–‘ Ι»ή“ΚΆ …ΪΓΘ«κ…ηΦΤ“ΜΗωΦρΒΞΒΡ Β―ιΖΫΑΗά¥≈–ΕœΦΉΓΔ““ΝΫΈΜΆ§―ßΒΡΥΒΖ® «Ζώ’ΐ»Ζ___________ΓΘ