题目内容
已知下列三个热化学方程式
①H2(g)+O2(g)===H2O(g) ΔH=-241.8 kJ·mol-1
②C(s)+O2(g)===CO2(g) ΔH=-393.5 kJ·mol-1
③C(s)+H2O(g)===CO(g)+H2(g) ΔH=+131 kJ·mol-1
写出表示碳燃烧生成CO和CO燃烧生成CO2的燃烧热的热化学方程式。
_________________________________________;
_________________________________________。
①H2(g)+O2(g)===H2O(g) ΔH=-241.8 kJ·mol-1
②C(s)+O2(g)===CO2(g) ΔH=-393.5 kJ·mol-1
③C(s)+H2O(g)===CO(g)+H2(g) ΔH=+131 kJ·mol-1
写出表示碳燃烧生成CO和CO燃烧生成CO2的燃烧热的热化学方程式。
_________________________________________;
_________________________________________。
C(s)+1/2O2(g)===CO(g) ΔH=-110.8 kJ·mol-1
CO (g)+1/2O2(g)===CO2(g) ΔH=-282.7 kJ·mol-1
CO (g)+1/2O2(g)===CO2(g) ΔH=-282.7 kJ·mol-1
考查盖斯定律的应用于
①+③得④C(s)+1/2O2(g)===CO(g) ΔH=-110.8 kJ·mol-1
②-④得:CO (g)+1/2O2(g)===CO2(g) ΔH=-282.7 kJ·mol-1
①+③得④C(s)+1/2O2(g)===CO(g) ΔH=-110.8 kJ·mol-1
②-④得:CO (g)+1/2O2(g)===CO2(g) ΔH=-282.7 kJ·mol-1

练习册系列答案
 备战中考寒假系列答案
备战中考寒假系列答案
相关题目
 O2(g)=
O2(g)= P4O10(s) △H=-738.5kJ·mol-1
P4O10(s) △H=-738.5kJ·mol-1 
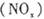 、二氧化碳等气体,常用下列方法处理,以实现节能减排、废物利用等。
、二氧化碳等气体,常用下列方法处理,以实现节能减排、废物利用等。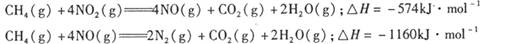
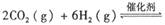
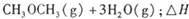 。已知在一定压强下,该反应随温度的升高而CO2的转化率降低。则该反应的
。已知在一定压强下,该反应随温度的升高而CO2的转化率降低。则该反应的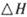 ________ 0(填“ >”或“ <”);若用以甲醚、空气、氢氧化钾溶液为原料,石墨为电极构成燃料电池,则该电池中负极的电极反应式是________________________________,放电过程中溶液的PH________ (填“增大”、“减小”或“不变”)。
________ 0(填“ >”或“ <”);若用以甲醚、空气、氢氧化钾溶液为原料,石墨为电极构成燃料电池,则该电池中负极的电极反应式是________________________________,放电过程中溶液的PH________ (填“增大”、“减小”或“不变”)。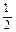 O2(g) =" CO" (g)
O2(g) =" CO" (g) 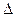 H1= —110.5kJ/mol
H1= —110.5kJ/mol