��Ŀ����
3����ͼ1��2Ϊ����ʵ��װ�ã�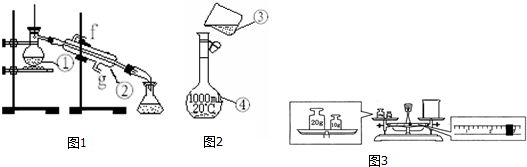
��1��д���������������ƣ���������ƿ���������ܣ�
��2�������١����У�ʹ��ʱ�������Ƿ�©ˮ���Тܣ�������ţ�
��3��������װ��ͼ1��ȡ����ˮ����ȱ�ٵ������Ǿƾ��ƣ��������������������ʵ�飬��ȴˮ��g�ڽ���
��4����������1.0mol•L-1��NaOH��Һ240mL������װ��ͼ2��ijͬѧ���ƴ���Һʱת�Ʋ�����ʾ��ͼ��ͼ������������ֱ���δ�ò�����������δ����250ml����ƿ��
��5��������Һʱ��һ����Է�Ϊ���¼������裺
�ٳ��� �ڼ��� ���ܽ� �ܵ�תҡ�� ��ת�� ��ϴ�� �߶��� ����ȴ
����ȷ�IJ���˳��Ϊ�ڢ٢ۢ�ݢޢߢܣ�
��6��ijͬѧ������һ������NaOH���壬������������ƽ�����ձ�����������ƽƽ����״̬��ͼ3���ձ���ʵ������Ϊ27.4g����ͬѧӦ����10.0g NaOH��
��7�������ƹ����У������������������ȷ�ģ����в���������Ũ��ƫ�ߵ��Ǣܣ�
��û��ϴ���ձ��Ͳ����� ��ת����Һʱ������������Һ��������ƿ���� ������ƿ�����������������ˮ �ܶ���ʱ���ӿ̶��� �ݶ���ҡ�Ⱥ�����Һ������ڿ̶��ߣ��ٲ�����������ˮ���̶��ߣ�
���� ��1����Ϥ��������״��˵�������ƣ�
��2��1000mL����ƿ��ʹ��ǰҪ����Ƿ�©ˮ��
��3����������ʵ���õ����������ƾ��ơ�ʯ������������ƿ���¶ȼơ������ܡ�ţ�ǹܡ���ƿ�����������ˮ�������¿ڽ��Ͽڳ���
��4������ƿֻ�����ù̶��������Һ���㵹��ҺӦ�ò�����������
��5����������һ�����ʵ���Ũ�ȵ���Һ����Ը�������������
��6���������̵�����=���̵�����+������������
����m=CVM����Ӧ��ȡ�������Ƶ�������
��7�������������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺��1��װ�â���������ƿ��װ�â��������ܣ��ʴ�Ϊ��������ƿ�������ܣ�
��2��1000mL����ƿ��ʹ��ǰҪ����Ƿ�©ˮ���ʴ�Ϊ���ܣ�
��3������ʵ���õ����������ƾ��ơ�ʯ������������ƿ���¶ȼơ������ܡ�ţ�ǹܡ���ƿ������ȱ�ٵ�����Ϊ�ƾ��ƣ�������ˮ�������¿ڽ��Ͽڳ���
�ʴ�Ϊ���ƾ��ƣ�g��
��4������һ�����ʵ���Ũ�ȵ���Һ�DZ����ò�������������ֹҺ���⽦�����ƶ���������Һ��ѡ����Ӧ��������ƿ��
�ʴ�Ϊ��δ�ò�����������δ����250mL����ƿ��
��5������һ�����ʵ���Ũ�ȵ���Һ����Ϊ�����㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ�������ȷ�IJ���˳��Ϊ���ڢ٢ۢ�ݢޢߢܣ�
�ʴ�Ϊ���ڢ٢ۢ�ݢޢߢܣ�
��6�������̵�����=���̵�����+�����������֪����������=�ձ�����+����������������ձ�����=��������-�������������ձ�����=20g+10g-2.6g=27.4g��ʵ����û��240mL����ƿ��ʵ�������Ƶ���250mL 1mol/L������������Һ����Ҫ�������Ƶ����ʵ���Ϊ��1mol/L��0.25L=0.25mol����Ҫ�������Ƶ�����Ϊ��40g/mol��0.25mol=10.0g��
�ʴ�Ϊ��27.4��10.0��
��7����û��ϴ���ձ��Ͳ��������������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ��ʲ�ѡ��
��ת����Һʱ������������Һ��������ƿ���棬�������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ��ʲ�ѡ��
������ƿ�����������������ˮ�������ʵ����ʵ�������Һ�����������Ӱ�죬��ҺŨ�Ȳ��䣬�ʲ�ѡ��
�ܶ���ʱ���ӿ̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ���ѡ��
�ݶ���ҡ�Ⱥ�����Һ������ڿ̶��ߣ��ٲ�����������ˮ���̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ��ʲ�ѡ��
��ѡ���ܣ�
���� ���⿼��������ʵ�飬һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ʵ���ԭ��������ʹ��ע���������һ�����ʵ���Ũ����Һ��ԭ���ǽ���ؼ�����Ŀ�ѶȲ���

 ����̼ѭ��������ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2��������ȫ������ձ����ӣ�
����̼ѭ��������ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2��������ȫ������ձ����ӣ�I�� �õ绡���ϳɵĴ�������̼�ܣ����������������ᴿ�������������Ӧ��
5 C+4 KMnO4+6 H2SO4��5CO2��+4MnSO4+2K2SO4+6 H2O
II��ij�о�С���ֽ�����CO��g����H2O��g���Ļ������ֱ�ͨ�����Ϊ2L�ĺ����ܱ������У�һ�������·�����Ӧ��CO��g��+H2O��g���TCO2��g��+H2��g����H��0���õ��������ݣ�
| ʵ���� | �¶�/�� | ��ʼ����mol�� | ƽ������mol�� | �ﵽƽ���� ��Ҫʱ��/min | ||
| CO��g�� | H2O��g�� | CO2��g�� | H2��g�� | |||
| I | 800 | 2 | 2 | x | 1 | 5 |
| II | 900 | 1 | 2 | 0.5 | 0.5 | tm |
| III | 900 | 2 | 4 | y | y | tn |
��2���������ж���800��ʵ��������CO��g����H2O��g����Ӧһ���ﵽƽ��״̬����D��
A��������ѹǿ���ٱ仯 B��$\frac{c��C{O}_{2}��•c��{H}_{2}��}{c��CO��•c��{H}_{2}O��}$=2
C����������ܶȲ��� D��������CO��=������CO2��
��3��ʵ����У�y=1��
��4����ʵ����������Ϊ�ھ��ȵ��ܱ������н��У�ʵ����H2O��g����ת����a��H2O����ʱ��仯��ʾ��ͼ����ͼ��ʾ��b�㣺���������������������=����������
| A�� | �¶����ߣ�Na2CO3��ҺpH��С | |
| B�� | ����������Һ�����ڿ����У���ҺpH��� | |
| C�� | ������ˮ������һ��ʱ�����ҺpH��С | |
| D�� | �¶����ߣ���ˮpH���� |
| A�� | 2Na+2NH3�T2NaNH2+H2�� | B�� | 2NH3+3CuO�T3Cu+N2+3H2O | ||
| C�� | NH3+H2O?NH3•H2O | D�� | HCl+NH3�TNH4Cl |
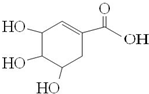 ����ơ���ʿ������ҩ��˾������һ�����������кͼ���H1N1���У������У�����Чҩ����Ƶ���Ҫ��Ч�ɷ�ç�����Ǵ��й����ճ����ĵ�ζ�ϰ˽���������ȡ�����ģ�ç����Ľṹʽ��ͼ������˵����ȷ���ǣ�������
����ơ���ʿ������ҩ��˾������һ�����������кͼ���H1N1���У������У�����Чҩ����Ƶ���Ҫ��Ч�ɷ�ç�����Ǵ��й����ճ����ĵ�ζ�ϰ˽���������ȡ�����ģ�ç����Ľṹʽ��ͼ������˵����ȷ���ǣ�������| A�� | ����ʹ��ˮ��ɫ | |
| B�� | ��FeCl3��Һ����ɫ | |
| C�� | �����ܷ�����ȥ��Ӧ | |
| D�� | 1molç������������Ʒ�Ӧ���Եõ�4molH2 |
| A�� | ƽ��������Ӧ�����ƶ��� | B�� | ����A��ת���ʼ����� | ||
| C�� | ����C���������������� | D�� | a+b��c |
| A�� | H2O | B�� | H2O2 | C�� | 11H��21H | D�� | 1940Ca��2040Ca |
 ���ײ���TiO2��Ϳ�ϡ��������ױƷ���������ż���㷺��Ӧ�ã��Ʊ�����TiO2�ķ���֮һ��TiCl4�ڼ���������ˮ������TiO2•xH2O���������ˡ�ˮϴ��ȥ���е�Cl-���پ���ɡ����ճ�ȥˮ�֣����õ�����TiO2��
���ײ���TiO2��Ϳ�ϡ��������ױƷ���������ż���㷺��Ӧ�ã��Ʊ�����TiO2�ķ���֮һ��TiCl4�ڼ���������ˮ������TiO2•xH2O���������ˡ�ˮϴ��ȥ���е�Cl-���پ���ɡ����ճ�ȥˮ�֣����õ�����TiO2��