جâؤ؟ؤعبف
،¾جâؤ؟،؟تض³ض¼¼تُµؤرُµç»¯ر§´«¸ذئ÷؟ةسأسع²â¶¨ O2؛¬ء؟£¬سزح¼خھؤ³ضضرُµç»¯ر§´«¸ذئ÷µؤشہيت¾زâح¼،£زرضھشع²â¶¨O2؛¬ء؟¹³جضذ£¬µç½âضتبـز؛µؤضتء؟±£³ض²»±ن،£ز»¶¨ت±¼نؤع£¬بôح¨¹´«¸ذئ÷µؤ´²âئّجهخھaL(±ê ×¼×´؟ِ)£¬ؤ³µç¼«شِضطءثb g،£دآءذثµ·¨²»صب·µؤتا£¨ £©

A.Ptµç¼«خھ¸؛¼«
B.·´س¦¹³جضذ×ھزئOH-µؤخïضتµؤء؟خھ0.125bmol
C.Pbµç¼«ةد·¢ةْµؤµç¼«·´س¦خھPb-2e- +2OH- = PbO+H2O
D.´²âئّجههّرُئّµؤجه»·ضتخھ![]()
،¾´ً°¸،؟B
،¾½âخِ،؟
¸ù¾ف×°ضأح¼·ضخِ؟ةضھ£¬¸أ³طخھشµç³ط£¬ح¨بëرُئّµؤز»¼«خھص¼«£¬ززµç¼«¼´Pbµç¼«خھ¸؛¼«£¬
A£®Ptµç¼«ح¨رُئّ£¬خھص¼«£¬ر،دîA´يخَ£»
B£®Pbµç¼«خھ¸؛¼«£¬ت§ب¥µç×س·¢ةْرُ»¯·´س¦£¬µç¼«·½³جت½خھ2Pb+4OH--4e-£½2PbO+2H2O£¬½ل؛د4molاâرُ¸ùہë×س£¬µç¼«ضتء؟شِضط32g£¬¸أµç¼«شِضطµؤضتء؟خھbg£¬شٍ×ھزئOH-µؤخïضتµؤء؟خھ0.125bmol£¬ر،دîBصب·£»
C£®Pbµç¼«خھ¸؛¼«£¬ت§ب¥µç×س·¢ةْرُ»¯·´س¦£¬µç¼«·½³جت½خھ2Pb+4OH--4e-£½2PbO+2H2O£¬ر،دîC´يخَ£»
D£®¸ù¾فر،دîBµؤ·ضخِ؟ةضھ£¬×ھزئµç×سخïضتµؤء؟خھ0.125bmol£¬سةµç¼«·½³جت½خھO2+2H2O+4e-£½4OH-£¬±ê×¼×´؟ِدآدû؛ؤرُئّµؤجه»خھ![]() =0.7bL£¬¹ترُئّµؤجه»·ضتخھ
=0.7bL£¬¹ترُئّµؤجه»·ضتخھ![]() £¬µ«أ»ثµأ÷±ê×¼×´؟ِ£¬ر،دîD´يخَ£»
£¬µ«أ»ثµأ÷±ê×¼×´؟ِ£¬ر،دîD´يخَ£»
´ً°¸ر،B،£

 جط¸ك¼¶½جت¦µم²¦دµءذ´ً°¸
جط¸ك¼¶½جت¦µم²¦دµءذ´ً°¸،¾جâؤ؟،؟أ؛»¯¹¤ضذ³£ذèرذ¾؟²»ح¬خآ¶بدآئ½؛â³£ت،¢ح¶ءد±ب¼°ببضµµبختجâ،£زرضھ£؛CO(g)+H2O(g)H2(g)+CO2(g)ئ½؛â³£تثوخآ¶بµؤ±ن»¯بçدآ±ي£؛
خآ¶ب/،و | 400 | 500 | 800 |
ئ½؛â³£تK | 9.94 | 9 | 1 |
تش»ط´ًدآءذختجâ£؛
£¨1£©ةدتِصدٍ·´س¦تا________·´س¦ (جî،°·إبب،±»ٍ،°خüبب،±)£»
£¨2£©دآءذجُ¼µؤ¸ؤ±نؤـ¼س؟ىئن·´س¦ثظآتµؤتا_______(ر،جîذٍ؛إ)£»
¢ظة¸كخآ¶ب£»¢ع±£³ضجه»²»±ن£¬ض»شِ¼سCOµؤضتء؟£»¢غ±£³ضجه»²»±ن£¬³نبëNeت¹جهدµر¹ا؟شِ´َ£»¢ـ±£³ضر¹ا؟²»±ن£¬³نبëNeت¹بفئ÷µؤجه»شِ´َ
£¨3£©شعز»بف»خھ2Lµؤأـ±صبفئ÷ؤع£¬³نبë0.2molCOسë0.4molH2£¬·¢ةْ·´س¦CO(g)+2H2(g) CH3OH(g)£¬COµؤئ½؛â×ھ»¯آتسëخآ¶ب£¬ر¹ا؟µؤ¹طدµبçدآح¼ثùت¾£؛
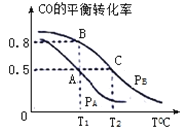
¢ظA،¢Bء½µم¶شس¦µؤر¹ا؟´َذ،¹طدµتاPA________PB£»(جî،°>،±£¬،°<،±£¬،°=،±)
¢عA،¢B،¢Cبµمµؤئ½؛â³£تKA،¢KB،¢KCµؤ´َذ،¹طدµتا ____________£»
¢غشعP1ر¹ا؟،¢T1،وت±£¬¸أ·´س¦µؤئ½؛â³£تK=___________£»
£¨4£©T1،و،¢1Lµؤأـ±صبفئ÷ؤع·¢ةْةدتِ·´س¦£¬²âµأؤ³ت±؟ج¸÷خïضتµؤخïضتµؤء؟بçدآ£؛CO£؛0.1mol H2£؛0.2mol CH3OH£؛0.2mol،£´ثت±vص ____ vؤو(جî >،¢< »ٍ =)،£