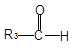��Ŀ����
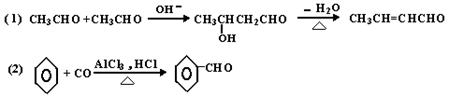
����ϩ����������������������100��ֵĸ߷��ӵ��壮�ɷ��ϳɵķ�Ӧ�ǣ�
(CH3)2C��O��HCN �� (CH3)2C(OH)CN
(CH3)2C(OH)CN��CH3OH��H2SO4 �� CH2��C(CH3)COOCH3�� NH4HSO4
90����·��ķ�Ӧ�ǣ�CH3C��CH�� CO�� CH3OH �� CH2��C(CH3)COOCH3
��ɷ��Ƚϣ��·����ŵ��ǣ�
(CH3)2C��O��HCN �� (CH3)2C(OH)CN
(CH3)2C(OH)CN��CH3OH��H2SO4 �� CH2��C(CH3)COOCH3�� NH4HSO4
90����·��ķ�Ӧ�ǣ�CH3C��CH�� CO�� CH3OH �� CH2��C(CH3)COOCH3
��ɷ��Ƚϣ��·����ŵ��ǣ�
| A��ԭ���ޱ�ըΣ�� | B��û�и����ԭ�������ʸ� |
| C��ԭ�϶��������� | D�����豸��ʴ�Խ�С |
BD
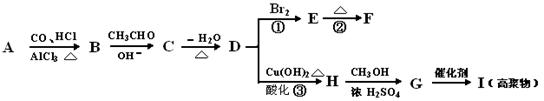
���������A���·���ԭ������CO��ȼ�����壬�б�ը���ޣ�����˵��ԭ���ޱ�ըΣ�ա�������CO�ж������Բ���˵��ԭ�϶��������ʡ�����A��C����C�����·��Ļ�ѧ����ʽ��������ֻд��һ�֣����ɷ��ķ�Ӧ��ѧ����ʽ�У����������֣�����֪�·����ŵ��ǡ�û�и����ԭ�������ʸߡ�����B��ȷ��D��Ա�������ѧ����ʽ����֪�·���ԭ����û��HCN��H2SO4���ʶ��豸��ʴ�Խ�С������D��ȷ��

��ϰ��ϵ�д�
 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д�
�����Ŀ
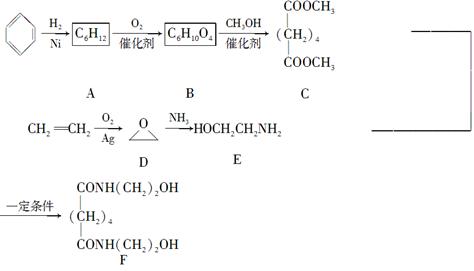
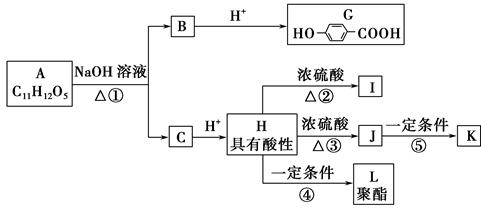
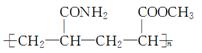 ��ͨ����ϩ���Ƶ�E���乤ҵ�ϳ�·����ͼ��ʾ��
��ͨ����ϩ���Ƶ�E���乤ҵ�ϳ�·����ͼ��ʾ��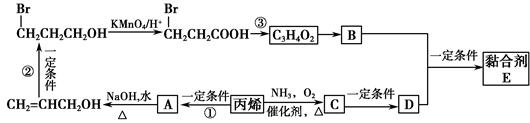
 2CH2=CHCN��6H2O
2CH2=CHCN��6H2O
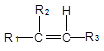
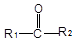 +
+