��Ŀ����
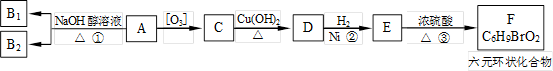
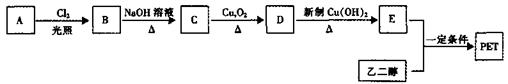
�л���A��һ�־��ô��ᵯ����Ҫ��Ч�ɷ֡�������A����Է�������Ϊ161��������C��HԪ���⣬��������һ��±��Ԫ�أ��ҷ�����ֻ����һ������������A��F��ת����ϵ����ͼ��ʾ����������������Cu(OH)2����Һ��1mol C��Ӧ������1mol Cu2O ��1mol D��B1��B2��Ϊ���ȶ��Ļ������һ�Ϊͬ���칹�塣
��֪����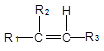
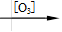
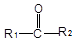 +
+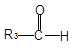
��һ��̼ԭ������������̼̼˫���Ľṹ����C��C��C�������ȶ���
������������⣻
��1��������A���еĹ������� ��B1����Է��������� ��
��2���١��ڡ��۵ķ�Ӧ���ͷֱ��� ��
��3��д��A��F�Ľṹ��ʽ��
A�� ��F�� ��
��4��д��C��D��Ӧ�Ļ�ѧ����ʽ��
��
��5����������������C��ͬ���칹�干��________�֡�
�ٺ��ж��������ں��ж���ȩ����

��֪����
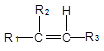
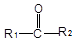 +
+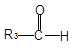
��һ��̼ԭ������������̼̼˫���Ľṹ����C��C��C�������ȶ���
������������⣻
��1��������A���еĹ������� ��B1����Է��������� ��
��2���١��ڡ��۵ķ�Ӧ���ͷֱ��� ��
��3��д��A��F�Ľṹ��ʽ��
A�� ��F�� ��
��4��д��C��D��Ӧ�Ļ�ѧ����ʽ��
��
��5����������������C��ͬ���칹�干��________�֡�
�ٺ��ж��������ں��ж���ȩ����
��1��̼̼˫���� -Br(��ԭ��)��80 (ÿ��1�֣���3��)
��2����ȥ��Ӧ���ӳɷ�Ӧ(��ԭ��Ӧ)��������Ӧ(��ȡ����Ӧ)��3�֣�ÿ��һ��1�֣�
��3��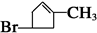 ��2�֣�
��2�֣� 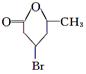 ��2�֣�
��2�֣�
��4��OHCCH2CHBrCH2COCH3��2Cu(OH)2��HOOCCH2CHBrCH2COCH3��Cu2O����2H2O ��2�֣�
��5��5 ��2�֣�
��2����ȥ��Ӧ���ӳɷ�Ӧ(��ԭ��Ӧ)��������Ӧ(��ȡ����Ӧ)��3�֣�ÿ��һ��1�֣�
��3��
 ��2�֣�
��2�֣�  ��2�֣�
��2�֣���4��OHCCH2CHBrCH2COCH3��2Cu(OH)2��HOOCCH2CHBrCH2COCH3��Cu2O����2H2O ��2�֣�
��5��5 ��2�֣�
���������������������ϢF�÷���ʽ��֪A����һ��Br��6��C������A����Է�������Ϊ161��AΪC6H9Br�������Ͷ�Ϊ2��A�ض��ǻ�״���������һ��˫����������ṹ����һ���ʻ������������Ƶ�
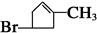 �� B1��B2�ֱ�Ϊ��
�� B1��B2�ֱ�Ϊ��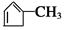 ��
��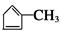 ��C��CH3-CO-CH2-CH(Br)-CH2-CHO��D��CH3-CO-CH2-CH(Br)-CH2-COOH��E��CH3-CH(OH)-CH2-CH(Br)-CH2-COOH��F��
��C��CH3-CO-CH2-CH(Br)-CH2-CHO��D��CH3-CO-CH2-CH(Br)-CH2-COOH��E��CH3-CH(OH)-CH2-CH(Br)-CH2-COOH��F�� 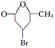
��1��������A���еĹ�������̼̼˫���� -Br(��ԭ��)��
 ��
��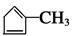 ������Ϊ80��
������Ϊ80����2���ֱ�Ϊ����ȥ��Ӧ���ӳɷ�Ӧ(��ԭ��Ӧ)��������Ӧ(��ȡ����Ӧ)��
��3��AΪ
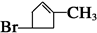 ��FΪ
��FΪ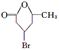 ��
����4����ӦΪOHCCH2CHBrCH2COCH3��2Cu(OH)2��HOOCCH2CHBrCH2COCH3��Cu2O����2H2O
��5��������������ΪOHC-C-C-CHO����������-CH3��һ��Br����5�����ӷ�ʽ��

��ϰ��ϵ�д�
 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�
�����Ŀ
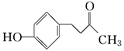 ����һ�ִ�ֲ�︲��������ȡ�Ľ�Ϊ��ȫ�����ϡ�һ����ʯ�ͻ�����ƷA�ͱ�ϩΪԭ�ϵĺϳ�·�����£�
����һ�ִ�ֲ�︲��������ȡ�Ľ�Ϊ��ȫ�����ϡ�һ����ʯ�ͻ�����ƷA�ͱ�ϩΪԭ�ϵĺϳ�·�����£�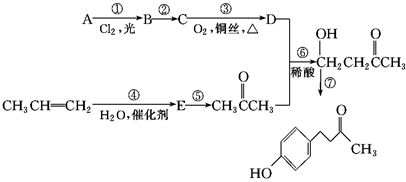
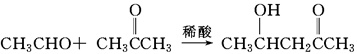
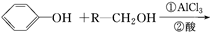

 ���ͱ�ͪ��
���ͱ�ͪ�� ���Ʊ�������
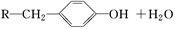
���Ʊ������� �ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�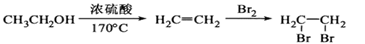
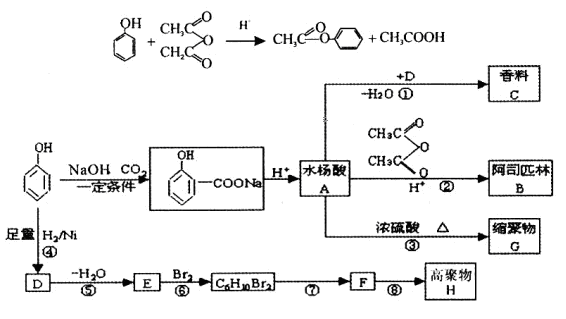

 �����ᣬ��B��ˮ����ﻥΪͬ���칹�塣H����FeCl3��Һ������ɫ��Ӧ���ұ����ϵ�һ�ȴ���ֻ��2�֡�д��������������������H�Ľṹ��ʽ��__________��
�����ᣬ��B��ˮ����ﻥΪͬ���칹�塣H����FeCl3��Һ������ɫ��Ӧ���ұ����ϵ�һ�ȴ���ֻ��2�֡�д��������������������H�Ľṹ��ʽ��__________��


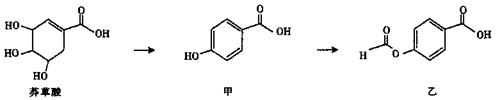
 Ϊԭ�Ϻϳ�
Ϊԭ�Ϻϳ�  ����ĵ�һ���ĺϳ�·�ߺ����һ���Ļ�ѧ����ʽ�����Լ���ѡ����
����ĵ�һ���ĺϳ�·�ߺ����һ���Ļ�ѧ����ʽ�����Լ���ѡ����