��Ŀ����
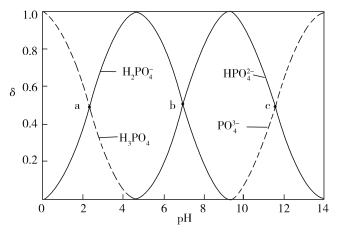
����Ŀ����ͼΪ����ʱ��ͬpH����������Һ�к�������̬�ķֲ�������a��b��c�����Ӧ��pH�ֱ�Ϊ2.12��7.21��11.31����������ʾ�����������ʵ�������������˵����ȷ����______
A��2 mol H3PO4��3 mol NaOH��Ӧ�����Һ������
B��NaOH��Һ�ζ�Na2HPO4��Һʱ�����÷�ָ̪ʾ�յ�
C��H3PO4�Ķ������볣����������Ϊ107
D����Һ�г�OH�����⣬����������Ũ�����ʱ����Һ���������ԡ����Ի����
���𰸡�![]()
��������
2 mol H3PO4��3 mol NaOH��Ӧ�����ɵ����ʵ���Ũ�ȵ�NaH2PO4��Na2HPO4����ͼ�ɿ���b��![]() ��
��![]() Ũ����ȣ�pH=7.21���Լ��ԣ�Aѡ�����NaOH��Һ����Na2HPO4��Һʱ��������Ӧ
Ũ����ȣ�pH=7.21���Լ��ԣ�Aѡ�����NaOH��Һ����Na2HPO4��Һʱ��������Ӧ![]() +OH
+OH![]()
![]() +H2O����(
+H2O����(![]() )������(
)��С����(![]() )�����ڴ�pH�仯��Χ�ڣ����÷�ָ̪ʾ�յ㣬Bѡ����ȷ��H3PO4
)�����ڴ�pH�仯��Χ�ڣ����÷�ָ̪ʾ�յ㣬Bѡ����ȷ��H3PO4![]() H++
H++![]() ��
��![]()
![]() H++
H++![]() ��Ka2=
��Ka2=![]() ����c(
����c(![]() )=c(
)=c(![]() )ʱ��pH=7.21��c(H+)=107.21��������Ϊ108��Cѡ�������Һ��������Ũ�����ʱ��������c(
)ʱ��pH=7.21��c(H+)=107.21��������Ϊ108��Cѡ�������Һ��������Ũ�����ʱ��������c(![]() )=c(
)=c(![]() )����ʱ��ҺpH=7.21�Լ��ԣ���������c(
)����ʱ��ҺpH=7.21�Լ��ԣ���������c(![]() )=c(
)=c(![]() )����ʱpH=11.31���Լ��ԣ�Dѡ�����
)����ʱpH=11.31���Լ��ԣ�Dѡ�����

����Ŀ������ˮ�Ǻϳ�ϩ���ij��÷�����ʵ���Һϳɻ���ϩ�ķ�Ӧ��ʵ��װ�����£�
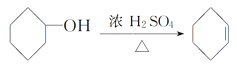 +H2O
+H2O
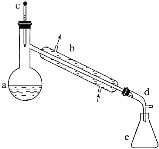
�����õ����й��������£�
��Է������� | �ܶ�/ g��cm��3 | �е�/�� | �ܽ��� | |
������ | 100 | 0.9618 | 161 | ����ˮ |
����ϩ | 82 | 0.8102 | 83 | ������ˮ |
�ϳɷ�Ӧ��
��a�м���20g��������2СƬ���Ƭ����ȴ��������������1mLŨ���ᡣb��ͨ����ȴˮ��ʼ��������a�������������¶Ȳ�����90�档
�����ᴿ��
��Ӧ�ֲ��ﵹ���Һ©���зֱ�������5%̼������Һ��ˮϴ�ӣ�����������ˮ�Ȼ��ƿ���������һ��ʱ�����ȥ�Ȼ��ơ�����ͨ������õ���������ϩ10g��
�ش��������⣺
��1��װ��b��������______��
��2���������Ƭ��������________���������һ��ʱ��������ǼӴ�Ƭ��Ӧ�ò�ȡ����ȷ������________(����ĸ����ͬ)��
A���������� B����ȴ�� C�����貹�� D����������
��3����ʵ���������ײ����ĸ�����Ľṹ��ʽΪ________________��
��4����Һ©����ʹ��ǰ����ϴ�ɾ���______���ڱ�ʵ���������У�����Ӧ�ôӷ�Һ©����_______(��Ͽڵ��������¿ڷų���)��
��5�������ᴿ�����м�����ˮ�Ȼ��Ƶ�Ŀ����______________��
��6���ڻ���ϩ�ֲ�����������У��������õ���������________��
A Բ����ƿ B �¶ȼ� C ����ƿ D ���������� E ������
��7����ʵ�����õ��Ļ���ϩ������________��
A��41% B��50% C��61% D��70%