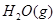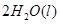��Ŀ����
��7�֣���1����ͬѧ��һ�ֱ�����ȥ�ⶨһ��δ֪Ũ�ȵİ�ˮ��Һ��Ũ�ȣ�Ӧѡ��___��___��ָʾ�����ζ��յ���Һ��ɫ�仯__��___�����ô���Һ��ϴ��ƿ��������İ�ˮ��ҺŨ�ȣ�������ƫ�ߣ�ƫ�ͣ���Ӱ����գ�___��__������ʽ�ζ�����������ˮϴ����û���ñ�������ϴ�������ⰱˮ����ҺŨ��__��___������ʽ�ζ��ܵζ�ǰ������ȷ���ζ����Ӷ����������ⰱˮ����ҺŨ��__��___��
��2��PH��ͬ�������������Һ�ֱ�������ˮϡ����ԭ����m ����n����ϡ�ͺ�����Һ��PH����ͬ����m __��___ n��ѡ��> ��< �� = ����������PH��ͬ�İ�ˮ������������Һ��Һ������������������m __��___ n��ѡ�� > ��< �� = ����
��2��PH��ͬ�������������Һ�ֱ�������ˮϡ����ԭ����m ����n����ϡ�ͺ�����Һ��PH����ͬ����m __��___ n��ѡ��> ��< �� = ����������PH��ͬ�İ�ˮ������������Һ��Һ������������������m __��___ n��ѡ�� > ��< �� = ����
��1�����ȣ��Ƶ��ȣ�ƫ��ƫ��ƫ�ͣ���2��m = n��m >n
�����������1�����ݷ�Ӧ��õ�ǿ�������Σ���Һ�����ԣ�Ӧ��ѡ�����Ա�ɫ��ָʾ���������ü��ȣ��ζ��յ����ɫ�仯Ϊ�Ƶ��ȡ�����
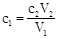 ���ô���ҹ��ϴ��ƿ�����ı�Һ�����������ֵ������ƫ�ߣ���ʽ�ζ���û����ϴ�����ı�Һ�����������ֵ������ƫ�ߣ��ζ�ǰ������ȷ���ζ����Ӷ���������������������ֵС����ƫ�͡�
���ô���ҹ��ϴ��ƿ�����ı�Һ�����������ֵ������ƫ�ߣ���ʽ�ζ���û����ϴ�����ı�Һ�����������ֵ������ƫ�ߣ��ζ�ǰ������ȷ���ζ����Ӷ���������������������ֵС����ƫ�͡���2����������ᶼ��ǿ�ᣬϡ��ǰpH��ͬ��ϡ�ͺ��pHҲ��ͬ������ϡ�͵ı���Ҳ��ͬ����m = n����ˮ�����NaOH��ǿ�ϡ��ǰpH��ͬ�����ϡ����ͬ��������ˮ���ڵ���ƽ�⣬����ϡ�ͺ�ˮ��pH��NaOH��Һ�Ĵ�Ҫ��ϡ�ͺ��pHҲ��ͬ����ˮ��Ҫˮ���������m >n��
�������ڽ����к͵ζ�������ʱ����Ҫ����
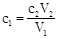 ������ʵ������У�V1��C2�Dz���ģ���˹ؼ��Ƿ�����������ε���V2�仯�ļ��ɡ�
������ʵ������У�V1��C2�Dz���ģ���˹ؼ��Ƿ�����������ε���V2�仯�ļ��ɡ�
��ϰ��ϵ�д�
 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�
�����Ŀ
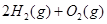 ===
===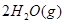
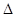 H="-483.6"
H="-483.6" 
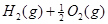 ===
===