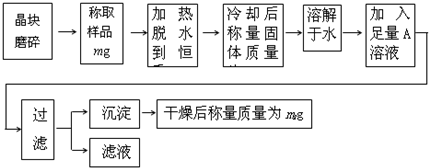
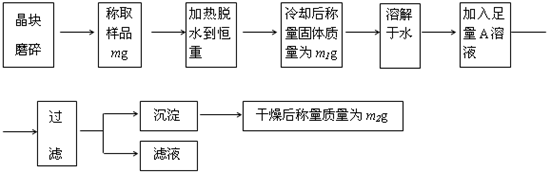
��Ŀ����
Ϊ�˲ⶨ����[KAl��SO4��?nH2O]�Ĵ��ȣ����л�������KCl���ӷ������ʣ���n��ֵ�����������̽���ʵ�飮
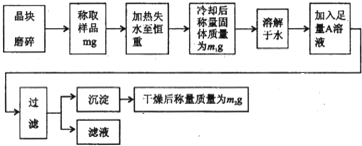
��1������ĥ��ʱʹ�õ����������� ��
��2���жϾ���ʧˮ�����صķ����� ��
��3������Һ��ֻ����һ�������ӣ�������ˮ�����0H-����A��Һ�� ���ѧʽ����
��4��ijͬѧ����ʵ��֤��������Ʒ�к��еĽ��������ӣ��������ͬѧ�������ʵ��������д�����ʣ�ಿ�֣�
��5�������ȹ������й���ɽ����������ʧ�����ʹ��õ�nֵ ���ƫ����ƫС����Ӱ�족����ͨ��ʵ�飬���������ѧʽ��nֵΪ12���������Ĵ���Ϊ %��

��1������ĥ��ʱʹ�õ�����������
��2���жϾ���ʧˮ�����صķ�����
��3������Һ��ֻ����һ�������ӣ�������ˮ�����0H-����A��Һ��
��4��ijͬѧ����ʵ��֤��������Ʒ�к��еĽ��������ӣ��������ͬѧ�������ʵ��������д�����ʣ�ಿ�֣�
| ������ | ʵ��������� | ʵ������ |
| K + | �ò�˿պȡ��������Һ���ھƾ��������գ�������ɫ�ܲ����۲� | |
| Al3+ | ʵ��������̣� |
���� ���ӷ���ʽ�� |
��������1��ʹ���в����������飻
��2��������ƽ��ȷ��0.1���ʵ����γ��������������0.1gʱ������Ϊ���������أ���ȫ��ˮ��
��3��������ͼ��֪��������Ʒ�Ĵ��ȣ��ɼ����Ȼ�����Һ�������������ᱵ�������ⶨ��Ʒ�Ĵ��ȣ���Һ�к��б�����֤����������ӳ�����ȫ��
��4���������������Ԫ�ؿ�֪�����������Ǽ����Ӻ������ӣ������ӿ���������ɫ��Ӧ���飬���������ú�����������Һ��Ӧ���ɰ�ɫ�����������������������Һ����������ܽ����ʵ�������֤��
��5�����������أ�������ɽ������²ⶨ��ˮ���������ⶨ�ᾧˮ���ʵ���ƫ�ߣ������������ᱵ�����������������������Ӷ�������Ʒ�Ĵ��ȣ�
��2��������ƽ��ȷ��0.1���ʵ����γ��������������0.1gʱ������Ϊ���������أ���ȫ��ˮ��
��3��������ͼ��֪��������Ʒ�Ĵ��ȣ��ɼ����Ȼ�����Һ�������������ᱵ�������ⶨ��Ʒ�Ĵ��ȣ���Һ�к��б�����֤����������ӳ�����ȫ��
��4���������������Ԫ�ؿ�֪�����������Ǽ����Ӻ������ӣ������ӿ���������ɫ��Ӧ���飬���������ú�����������Һ��Ӧ���ɰ�ɫ�����������������������Һ����������ܽ����ʵ�������֤��
��5�����������أ�������ɽ������²ⶨ��ˮ���������ⶨ�ᾧˮ���ʵ���ƫ�ߣ������������ᱵ�����������������������Ӷ�������Ʒ�Ĵ��ȣ�
����⣺��1��ʹ���в����������飬�ʴ�Ϊ���в���
��2��������ƽ��ȷ��0.1���ʵ����γ��������������0.1gʱ������Ϊ���������أ���ȫ��ˮ��
�ʴ�Ϊ�����γ������������0.1 g��
��3��������Ʒ�Ĵ��ȣ�����Һ��ֻ����һ�������ӣ��ɼ����Ȼ�����Һ�������������ᱵ�������ⶨ��Ʒ�Ĵ��ȣ���Һ�к��б�����֤����������ӳ�����ȫ��
�ʴ�Ϊ��BaCl2��
��4�������������Ԫ�ؿ�֪�����������Ǽ����Ӻ������ӣ������ӿ���������ɫ��Ӧ���飬���������ú�����������Һ��Ӧ���ɰ�ɫ�����������������������Һ����������ܽ����ʵ�������֤���ò�˿պȡ��������Һ���ھƾ��������գ�������ɫ�ܲ����۲������ɫ����֤�����м����ӣ���ô�����Һ����������������Һ�������������ְ�ɫ���������ɺͳ����ܽ�֤�����������ӣ���Ӧ�����ӷ���ʽΪAl3++3OH-=Al��OH��3����Al��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ����ɫ���棻��ô�����Һ����������������Һ�������������ɰ�ɫ����������ɫ�����ܽ⣻Al3++3OH-=Al��OH��3����Al��OH��3+OH-=AlO2-+2H2O��
��5�������ȹ������й���ɽ����������ʧ�����������أ����²ⶨ��ˮ���������ⶨ�ᾧˮ���ʵ���ƫ�ߣ�nƫ�ߣ�������������ᱵ������Ϊm2g�������ᱵ�����ʵ���Ϊ
=
mol����KAl��SO4��2?12H2O������Ϊ
mol��
��474g/moL=
g�����������Ĵ���Ϊ
��100%=
%���ʴ�Ϊ��ƫ��
��
��2��������ƽ��ȷ��0.1���ʵ����γ��������������0.1gʱ������Ϊ���������أ���ȫ��ˮ��
�ʴ�Ϊ�����γ������������0.1 g��
��3��������Ʒ�Ĵ��ȣ�����Һ��ֻ����һ�������ӣ��ɼ����Ȼ�����Һ�������������ᱵ�������ⶨ��Ʒ�Ĵ��ȣ���Һ�к��б�����֤����������ӳ�����ȫ��
�ʴ�Ϊ��BaCl2��
��4�������������Ԫ�ؿ�֪�����������Ǽ����Ӻ������ӣ������ӿ���������ɫ��Ӧ���飬���������ú�����������Һ��Ӧ���ɰ�ɫ�����������������������Һ����������ܽ����ʵ�������֤���ò�˿պȡ��������Һ���ھƾ��������գ�������ɫ�ܲ����۲������ɫ����֤�����м����ӣ���ô�����Һ����������������Һ�������������ְ�ɫ���������ɺͳ����ܽ�֤�����������ӣ���Ӧ�����ӷ���ʽΪAl3++3OH-=Al��OH��3����Al��OH��3+OH-=AlO2-+2H2O��
�ʴ�Ϊ����ɫ���棻��ô�����Һ����������������Һ�������������ɰ�ɫ����������ɫ�����ܽ⣻Al3++3OH-=Al��OH��3����Al��OH��3+OH-=AlO2-+2H2O��
��5�������ȹ������й���ɽ����������ʧ�����������أ����²ⶨ��ˮ���������ⶨ�ᾧˮ���ʵ���ƫ�ߣ�nƫ�ߣ�������������ᱵ������Ϊm2g�������ᱵ�����ʵ���Ϊ
| m2g |
| 233g/mol |
| m2 |
| 233 |
| m2 |
| 233 |
| 1 |
| 2 |
| 237m2 |
| 233 |
| ||
| mg |
| 23700m2 |
| 233m |
| 23700m2 |
| 233m |
���������⿼�����ʵ���ɺͺ����IJⶨ����Ŀ���ѣ�����ע��ʵ���ԭ���Ͳ���������ע�������Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ�������⡢��������������

��ϰ��ϵ�д�
�����Ŀ