��Ŀ����
6����4molA�����2molB������2L�������л�ϣ���һ�������·������·�Ӧ��2A��g��+B��g��?2C��g��������2s����C��Ũ��Ϊ0.6mol•L-1���������м���˵������������A��Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ0.3mol•L-1•s-1��
��2sʱ����A��ת����Ϊ70%��
��2sʱ����B��Ũ��Ϊ0.7mol•L-1��
����A��B�����ʵ���֮�Ȳ��ٱ仯ʱ����Ӧ�ﵽƽ�⣬
����������ѹǿ���ٱ仯ʱ����Ӧ�ﵽƽ�⣬
������������ͬ����������Ҳ��ͬ������£�����4molA�����4molB���壬��C��Ũ��Ϊ0.6mol•L-1ʱ������Ҫ��ʱ�����2s��
������ȷ���ǣ�������
| A�� | �٢ۢ� | B�� | �٢ܢ� | C�� | �ڢۢ� | D�� | �ۢܢ� |
���� �� 4mol A ����� 2mol B ������ 2L ���ܱ������л�Ϸ�����Ӧ������ 2s���룩���� C ��Ũ��Ϊ0.6mol•L-1 ������CΪ0.6mol•L-1 ��2L=1.2mol����
2A��g��+B��g��?2C��g��
��ʼ����mol����4 2 0
�仯����mol����1.2 0.6 1.2
2sĩ��mol����2.8 1.4 1.2
�ٸ���v=$\frac{��c}{��t}$����v��A����
��ת����=$\frac{���ʵ����仯��}{��ʼ���ʵ���}$��100%��
�۸���c=$\frac{n}{V}$����2sĩ����B��Ũ�ȣ�
��A��B��ʼ���ʵ���֮��Ϊ2��1�����ڻ�ѧ������֮�ȣ���Ӧ�ж������ʵ���֮��ʼ��Ϊ2��1��
���淴Ӧ���У��������ʵ�����С�����º�����ѹǿ��С����������ѹǿ���䣬˵������ƽ�⣻
��B��Ũ������Ӧ���ʼӿ죮
��� �⣺�� 4mol A ����� 2mol B ������ 2L ���ܱ������л�Ϸ�����Ӧ������ 2s���룩���� C ��Ũ��Ϊ0.6mol•L-1 ������CΪ0.6mol•L-1 ��2L=1.2mol����
2A��g��+B��g��?2C��g��
��ʼ����mol����4 2 0
�仯����mol����1.2 0.6 1.2
2sĩ��mol����2.8 1.4 1.2
��2s��v��A��=$\frac{\frac{1.2mol}{2L}}{2s}$=0.3mol/��L��s�����ʢ���ȷ��
��2sʱA��ת����=$\frac{1.2mol}{4mol}$��100%=30%���ʢڴ���
��2sĩ����B��Ũ��Ϊ$\frac{1.4mol}{2L}$=0.7mol/L���ʢ���ȷ��
��A��B��ʼ���ʵ���֮��Ϊ2��1�����ڻ�ѧ������֮�ȣ���Ӧ�ж������ʵ���֮��ʼ��Ϊ2��1���������ʵ���֮�Ȳ��䣬����˵������ƽ�⣬�ʢܴ���
���淴Ӧ���У��������ʵ�����С�����º�����ѹǿ��С����������ѹǿ���䣬˵������ƽ�⣬�ʢ���ȷ��
��B��Ũ������Ӧ���ʼӿ죬��C��Ũ��Ϊ0.6mol•L-1ʱ������Ҫ��ʱ��С��2s���ʢ���
��ѡ��A��
���� ���⿼�黯ѧƽ����㡢ƽ��״̬�жϡ���Ӧ����Ӱ�����صȣ�ע���ж�ƽ���������Ӧ�淴Ӧ���з����仯�����������ɱ仯�����ٱ仯˵������ƽ�⣮

| A�� | 0.1mol/LNa2S��Һ������S2-����С��O.1NA | |
| B�� | ��CH3COONa��Һ��CH3COO-����ĿΪNA����Na+����Ŀ����NA | |
| C�� | һ�������£�1mol N2��3 mol H2��ϳ�ַ�Ӧ��ת�Ƶĵ�����ĿΪ6NA | |
| D�� | ��״���£�11.2 L�����к��еĻ�ѧ����ĿΪ9.5NA |
| A�� | ϡ������������ͭ��Ӧ��H++OH-�TH2O | |
| B�� | ����ˮ��Ӧ��Na+H2O�TNa++OH-+H2�� | |
| C�� | ̼��������ᷴӦ��CO32 -+2H+�TH2O+CO2�� | |
| D�� | ̼��������CO32-+2H+�TH2O+CO2�� |
| �� �� | �� | �� |
| ��Ӧ��Ͷ���� | 3mol H2��2mol N2 | 6mol H2��4mol N2 |
| �ﵽƽ���ʱ�䣨min�� | t | 5 |
| ƽ��ʱN2��Ũ�ȣ�mol•L-1�� | 3 | c |
��2���������ﵽƽ������Ҫ��ʱ��t=5min�����������������=������
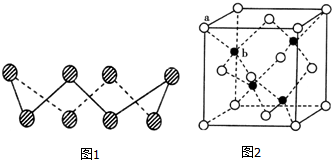 ��A�������������Se�����ڣ�Te����Ԫ���ڻ������г����ֳ���������̬������A��Ԫ�صĻ��������о�����������������Ҫ��;����ش��������⣺
��A�������������Se�����ڣ�Te����Ԫ���ڻ������г����ֳ���������̬������A��Ԫ�صĻ��������о�����������������Ҫ��;����ش��������⣺