��Ŀ����
��11�֣���1����������(Na2FeO4)��ˮ���������е�һ�����;�ˮ�������������Աȸ�����ظ�ǿ�������ڷ�Ӧ�б���ԭ�����������Ӵﵽ��ˮ��Ŀ�ġ����������������������ƺ�������һ���������Ƶõģ�ͬʱ�����������ƺ��Ȼ��Ƶȡ�
��д����ȡ�������ƵĻ�ѧ����ʽ�� ��
������ȡ�������Ƶķ�Ӧ�� Ԫ�ر�������
�۸�������֮�����о�ˮ���ã���ɱ���⣬��һ��ԭ���� ��
��2����ij�����������У�Ҫ�õ�һ����ɫ��������ˮ�ľ�����立����þ�����һ�ָ��Σ�����Ҫ��ѧ�ɷ�Ϊʮ��ˮ��������李���ø��ε�Ũ��Һ����μ���Ũ����������Һ��������һϵ�б仯����֪��NH4+��AlO2����ˮ��Һ�в��ܴ������棬�ᷢ�����·�Ӧ��NH4++A1O2��+H2O=Al(OH)3��+NH3��
�Իش�
�����������ˮ��Һ�еĵ��뷽��ʽΪ ��
������μ���Ũ����������Һ�Ĺ����У������������У�a��Һ�г��ְ�ɫ������b�д̼��������ݳ���c��ɫ�����������ࣻd��ɫ������ȫ��ʧ��e��ɫ�������١�
��ش�����������ȵ�����ֵ�˳����(����Żش�)�� ��
�۲�����ͼ�л����������Ƶļ��������������Ĺ�ϵ��
(1)
��2Fe(NO3)3+16NaOH+3Cl2=2Na2FeO4+6NaNO3 +6NaCl + 8 H2O
����Ԫ��
��FeO42-����ԭΪ�����ӣ�������ˮ�����ɵ���������������к�ǿ�������ԣ��ﵽ��ˮ�����á�
(2)
��NH4Al(SO4)2=NH4+ + Al3++ 2SO42��
��acbed
��
����:

 һ����������ϵ�д�
һ����������ϵ�д�Ŀǰ��������(Na2FeO4)���㷺Ӧ����ˮ���������и�Ч�������ŵ㡣
(1)�ڴ���ˮ�Ĺ����У�Na2FeO4��ɱ������������ˮ�����ã���˵��Ӧ����Na2FeO4����Щ���ʣ�__________________________________________________________________________
(2) ij�غ�ˮ��Ʒ��Na2FeO4�������������Ӽ���Ũ�����±���ʾ(H����OH��δ�г�)��
| ���� | SO | Mg2�� | Fe3�� | Na�� | Cl�� |
| Ũ��(mol/L) | a | 0.05 | 0.10 | 0.50 | 0.58 |
�����£�ȡһ������Na2FeO4�������ĺ�ˮΪԭ���Ʊ�����ʳ��ˮ��MgCl2��7H2O���������£�
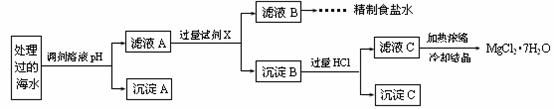
Ksp[Fe(OH)3]��1.0��10��38��Ksp[Mg(OH)2]��5.0��10��12��������������Һ����ı仯���Բ��ơ�
�� �����е�a________0.16(�<������>������)��
�� ����A�����Ϊ________(�ѧʽ)���ڵ�����ҺpHʱ��������Ӧ���ڵ�pH�ķ�Χ��________��
�� ����Ĺ����Լ�XΪ____________________(�ѧʽ)��
�� �������HCl������Ϊ_________________________________________________��
��1����Fe��OH��3��NaClO��Һ��ϣ����Ƶ�Na2FeO4����ɲ���ƽ�������ӷ���ʽ
______Fe��OH��3+______ClO-+______=______FeO42-+______Cl-+______
��2��ij�غ�ˮ��Ʒ��Na2FeO4�������������Ӽ���Ũ�����±���ʾ��H+��0H-δ�г�����
| ���� | SO42- | Mg2+ | Fe3+ | Na+ | Cl- |
| Ũ�ȣ�mol/L�� | a | 0.05 | 0.10 | 0.50 | 0.58 |
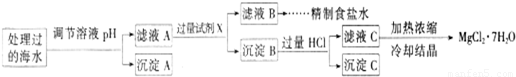
ע�����ӵ�Ũ��С��1.0×10-5mol/L������Ϊ�����Ӳ����ڣ�Ksp[Fe��OH��3]=1.0×10-38 Ksp[Mg��OH��2]=5.0×10-12��������������Һ����仯���Բ��ƣ�
�ٱ����е�a______0.16 �����������������=������
�ڳ���A�����Ϊ______ ���ѧʽ��
�ۼ���Ĺ����Լ�XΪ______ ���ѧʽ��
�ܼ������HCl������Ϊ______��

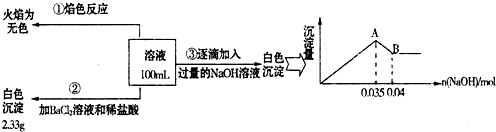

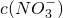 =______���������ڣ����ʲ������𣩣�
=______���������ڣ����ʲ������𣩣�