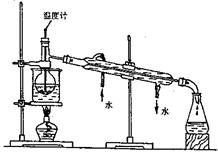
��Ŀ����
�ô����˫��ˮ��Ͽ���������Һ��ϴ�Ӽ�(2Na2CO3��3H2O2)��������ɱ������ȥ���۵������Ҳ�����ȾˮԴ��
(1)������������ϴ�Ӽ��н��������ӵIJ�����������_______________________________________��
(2)����ϴ�Ӽ��е�˫��ˮ���Խ���ˮ�е��軯��ת��Ϊ����ͬʱ����NH3��д����Ӧ�����ӷ���ʽ��___________________________________
(3)�������ϴ�Ӽ���ˮ�к��������ӣ�����������ϴ�Ӽ���ȥ��������������ȫʧȥɱ�����á��Է������е�ԭ��(�����ӷ���ʽ�ͼ�Ҫ���ֱ���)��__________________________________________________________________
(4)ij��ѧѧϰС��Ϊ����̽�������Ӷ���������ϴ�Ӽ��IJ���Ӱ�죬ȡ��ϴ�Ӽ�100 mL������25 gFeCl3���壬����������ɫ��ζ���壬������ƿ�ռ����塣��ѡ�������Լ���ʵ����Ʒ�������ɷֵ�̽�����̣�0.10 mol��L��1 NaOH��Һ��8.0 mol��L��1 NaOH��Һ������ʯ��ˮ��0.01 mol��L��1 KMnO4��Һ��BaCl2ϡ��Һ��Ʒ����Һ������ˮ��Сľ�����ƾ��ơ����ϴ��ƿ��
��������裺�Ը�����ɷ�����������衣
����1��������O2��
����2��������________________��
����3��������CO2��
����Ʒ��������ʵ�鷽��֤����ļ��裬���±������ʵ�鲽�衢Ԥ����������ۡ�
| ʵ�鲽�� | Ԥ����������� |
| ����������ͨ��ʢ��________��________��ϴ��ƿ�У�________ | __________________ |
��(1)�ýྻ�IJ�˿պȡϴ�Ӽ��ھƾ��ƻ��������գ�����ʻ�ɫ(��������)
(2)H2O2��CN����H2O=HCO3����NH3
(3)2H2O2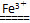 2H2O��O2���������ӻ����H2O2�ֽ⣬ʹϴ�Ӽ�ʧȥɱ�����ã�2Fe3����3CO32����3H2O=2Fe(OH)3����3CO2����Fe3����CO32��ˮ����ٽ���ʹϴ�Ӽ�ʧȥȥ������
2H2O��O2���������ӻ����H2O2�ֽ⣬ʹϴ�Ӽ�ʧȥɱ�����ã�2Fe3����3CO32����3H2O=2Fe(OH)3����3CO2����Fe3����CO32��ˮ����ٽ���ʹϴ�Ӽ�ʧȥȥ������
(4)��CO2��O2
��ʵ�鲽�� Ԥ����������� ����ʯ��ˮ
8.0 mol��L��1 NaOH��Һ�����������ǵ�Сľ���������һ��ϴ��ƿ�ij��ڴ�������ʯ��ˮ������ǣ�ľ����ȼ�������1������������ʯ��ˮ����ǣ�ľ����ȼ�������2������������ʯ��ˮ����ǣ�ľ������ȼ�������3����
����

 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д� �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д���ˮ�Ǿ����Դ���⣬��ҵ�ϴӺ�ˮ����ȡʳ�κ���Ĺ������£�
��1�������õ���-KI��ֽ�������I���Ƿ��������ɣ������������___________________��
��2������������Ӧ�����ӷ���ʽΪ_____________��������̼������Һ����������еĶ�������ˮ��Һ������������Ļ��ϼ۷ֱ�Ϊ+5��-1�ۣ��������������ϡ���������������������з�����Ӧ�����ӷ���ʽΪ______��
��3���屽��һ�ֻ���ԭ�ϣ���������ͱ���Ӧ�ϳɡ�ʵ���Һϳ��屽��װ��ʾ��ͼ���£�

�±�Ϊ��������屽��������ݣ�
| | �� | �� | �屽 |
| �ܶ�/g��cm-3 | 0.88 | 3.10 | 1.50 |
| �е�/�� | 80 | 59 | 156 |
| ˮ���ܽ�� | �� | �� | �� |
�ش��������⣺
��4����A�м���30 .0mL��ˮ����������м����B��С�ļ���8.0 mLҺ̬�塣��A�еμ�Һ�壬��Ӧһ��ʱ���ȼ�ƾ��Ƽ�������
���ڸ�ʵ���У� Bװ�õ�������__________��A���ݻ����ʺϵ���_________(����)
a��25mL b��50mL c��100mL d��250mL
���ռ��屽ʱ��Ӧ����_________(�C1����C2��)����ʾ���¶ȣ����¶�ӦΪ_________��
���ڷ�Ӧ��ʼ���ռ��屽֮ǰ��Ӧ�Ƚ�Fװ��������Dװ�ú������ɳнӵ�������_______________________(�ѧʽ)��
��ij��ѧС���������ʵ�鷽����֤�������巢������ȡ����Ӧ�����Ǽӳɷ�Ӧ��
��һ����ȡ������Ӧ��Fװ���е���Һ���Թ��У��ڶ����������м��������ϡ����������������μ���������������Һ�������dz��ɫ����������֤�������巢������ȡ����Ӧ��
��ʵ�鷽��__________(���������������)��������__________________��
��ǹ�ҵ���Ʊ�Na2S2O3�ķ���֮һ����Ӧԭ��Ϊ��2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2 ���÷�Ӧ��H>0��
ij�о�С����ʵ��������Ʊ�Na2S2O3��5H2O�������¡�
��1������װ����ͼ��ʾ��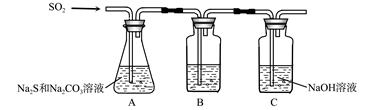
��װ��B�������Ǽ���װ��A��SO2������Ч�ʣ�B���Լ��� ������SO2����Ч�ʵ͵�ʵ��������B����Һ ��
��Ϊ��ʹSO2������������ȫ���ڲ��ı�A����ҺŨ�ȡ�����������£����˼�ʱ���跴Ӧ���⣬���ɲ�ȡ�ĺ�����ʩ�� �� ����д��������
��2�����豾ʵ�����õ�Na2CO3������NaCl��NaOH�����ʵ�鷽�����м��顣������ʱCaCO3������Һ��pH=10.2��
��ѡ�Լ���������ϡ���ᡢAgNO3��Һ��CaCl2��Һ��Ca��NO3��2��Һ����̪��Һ������ˮ��pH�ơ��ձ����Թܡ��ι�
| ��� | ʵ����� | Ԥ������ | ���� |
| �� | ȡ������Ʒ���Թ��У�������������ˮ��������ܽ⣬___________________�� | _______________ | ��Ʒ��NaCl |
| �� | ��ȡ������Ʒ���ձ��У�������������ˮ����ֽ����ܽ⣬___________________�� | _______________ | ��Ʒ��NaOH |
��3��Na2S2O3��Һ�Ƕ���ʵ���еij����Լ����ⶨ��Ũ�ȵĹ������£�ȷ��ȡa g KIO3����ѧʽ����214�����������Һ���������KI�����H2SO4��Һ���μ�ָʾ������Na2S2O3��Һ�ζ����յ㣬����Na2S2O3��Һ�����ΪV mL����c��Na2S2O3����_________mol��L��1����ֻ�г���ʽ���������㣩
��֪��IO3����5I��+6H+== 3I2��3H2O 2S2O32����I2==S4O62����2I��
������㷺Ӧ������ҩ�ͻ�����ҵ��ijͬѧ�����üױ���������Ӧ�Ʊ������ᡣ��Ӧԭ��: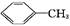 +2KMnO4
+2KMnO4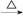
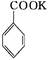 +KOH+
+KOH+
2MnO2��+H2O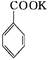 +HCl
+HCl
 +KCl
+KCl
ʵ�鷽��:һ�����ļױ���KMnO4��Һ��100 �淴Ӧһ��ʱ���ֹͣ��Ӧ,���������̷����������ͻ���δ��Ӧ�ļױ���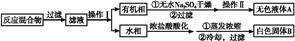
��֪:�����������122,�۵�122.4 ��,��25 ���95 ��ʱ�ܽ�ȷֱ�Ϊ0.3 g��6.9 g;���������л���һ�㶼�й̶��۵㡣
(1)��������������,����������������
(2)��ɫҺ��A����������,���Լ���A���Լ�����������,�������� ��
(3)�ⶨ��ɫ����B���۵�,��������115 �濪ʼ�ۻ�,�ﵽ130 ��ʱ�����������ۡ���ͬѧ�Ʋ��ɫ����B�DZ�������KCl�Ļ����,��������·��������ᴿ�ͼ���,ʵ���������Ʋ���ȷ������ɱ������ݡ�
| ��� | ʵ�鷽�� | ʵ������ | ���� |
| �� | ����ɫ����B����ˮ��,�����ܽ�,������ | �õ���ɫ�������ɫ��Һ | |
| �� | ȡ������Һ���Թ���,�������� | ���ɰ�ɫ���� | ��Һ��Cl- |
| �� | �����ɫ����,������ | ������ | ��ɫ���� �DZ����� |
(4)���Ȳⶨ:��ȡ1.220 g��Ʒ,���100 mL�״���Һ,��ȡ25.00 mL��Һ,�ζ�,����KOH�����ʵ���Ϊ2.40��10-3 mol����Ʒ�б��������������ļ������ʽΪ��������,������Ϊ��������(������λ��Ч����)��
�Ҷ���(H2C2O4)�׳Ʋ��ᣬ��һ����Ҫ�Ļ���ԭ�ϡ��������ϣ��˽�����й���Ϣ��
���Ҷ���������ˮ��������100�濪ʼ������125��ʱѸ��������157��ʱ������������ʼ�ֽ⡣�Ҷ������ȷֽ�����ˮ��������̼��һ�ֳ����Ļ�ԭ�����塣
���Ҷ���ĸ��Ρ����Ҷ����Ϊ������ˮ�İ�ɫ���塣
(1)��д���Ҷ������ȷֽ�Ļ�ѧ����ʽ______________________��
(2)��ѧ��ȤС���ͬѧ��ʵ��֤���Ҷ��ᾧ�����ȷֽ����ɵ�����ɷ֡�����������ͼ�ṩ��װ�ã���ѡ�Լ������������ʵ�鷽������A��B��C��C��C��D��E˳�������������װ�ã������Ҷ��ᾧ�����ȷֽ����ɵ�����ɷ֡�
���㰴����װ�ô������ҵ�˳����д�±��еĿո�
| �������� | �������������� | װ������ |
| B | | |
| C | | |
| C | ��������Ũ��Һ | |
| C | | |
| D | | |
| E | | |
(3)����ʵ������˵���Ҷ������ȷֽ������˻�ԭ�������ʵ��������________________________________________________________________��
(4)�����Ҷ�����н�ǿ�Ļ�ԭ�ԣ�ͨ��ѡ�õ��Լ���_______________��
TiO2�����Ʊ��������ѻ������ԭ�ϣ�����һ����������İ�ɫ���ϡ�
(1)ʵ�������÷�ӦTiO2(s)��CCl4(g)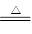 TiCl4(g)��CO2(g)������ˮ�����������Ʊ�TiCl4��ʵ��װ��ʾ��ͼ���£�
TiCl4(g)��CO2(g)������ˮ�����������Ʊ�TiCl4��ʵ��װ��ʾ��ͼ���£�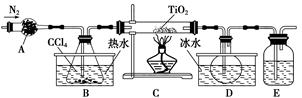
�й������������±���
| ���� | �۵�/�� | �е�/�� | ���� |
| CCl4 | ��23 | 76 | ��TiCl4���� |
| TiCl4 | ��25 | 136 | ����ʪ������������ |
(2)��ҵ����������(FeTiO3)(��Fe2O3��SiO2������)�Ʊ�TiO2���йط�Ӧ������
���ܡ�FeTiO3(s)��2H2SO4(aq)=FeSO4(aq)��TiOSO4(aq)��2H2O(l)
ˮ�⡡TiOSO4(aq)��2H2O(l)
 H2TiO3(s)��H2SO4(aq)
H2TiO3(s)��H2SO4(aq)��Ҫ�����������£�

���Լ�AΪ________����Һ������ȴ��70 �����ң����¶ȹ��ᵼ�²�ƷTiO2���ʽ��ͣ�ԭ����_________________________________________________
_______________________��
��ȡ������ϴ���H2TiO3���������Ტ���μ�KSCN��Һ�������������ټ�H2O2�������ɫ��˵��H2TiO3�д��ڵ�����������________������H2TiO3��ʹ��ˮ���ϴ�ӣ����պ��õ�TiO2Ҳ�ᷢ�ƣ����Ƶ�������____________________(�ѧʽ)��
�뵼�������г���Ҫ���Ʋ���,�Ա�֤���Ƶ�����,���Ȼ���(PCl3)��һ����Ҫ�IJ��Ӽ���ʵ����Ҫ�û���(����)������Cl2ģ�ҵ������ȡPCl3,װ������ͼ��ʾ:(���ּг�װ����ȥ)
��֪����������Cl2��Ӧ����PCl3,�����Cl2��Ӧ����PCl5��PCl3��ˮ��ǿ��ˮ������H3PO3��HCl,��O2������POCl3,POCl3����PCl3��PCl3��POCl3���۷е���±�:
| ���� | �۵�/�� | �е�/�� |
| PCl3 | -112 | 75.5 |
| POCl3 | 2 | 105.3 |
��ش���������:
(1)Aװ���������������ӷ���ʽΪ��������������������������
(2)B����װ�Լ�������������,E����ˮ������������������������,F�м�ʯ�ҵ�����������������������������
(3)ʵ��ʱ,���װ�������Ժ�,�ȴ�K3ͨ������CO2,��Ѹ�ټ�����ס�ͨ�����CO2����������������������������������ͨ������K1��K2�ܳ�ȥA��Bװ���еĿ���,����ķ���������������������������������������������
(4)�ֲ�Ʒ�г�����POCl3��PCl5�ȡ���������ȳ�ȥPCl5��,ͨ������������(��ʵ���������),���ɵõ��ϴ�����PCl3��