题目内容
【题目】对工业废水和生活污水进行处理是防止水体污染、改善水质的主要措施之一。
(1)硫酸厂的酸性废水中砷(As)元素(主要以 H3AsO3 形式存在)含量极高,为控制砷的排放, 某工厂采用化学沉淀法处理含砷废水。请回答以下问题:
①已知砷是氮的同族元素,比氮原子多 2 个电子层,砷在元素周期表的位置为_____。
②工业上采用硫化法(通常用硫化钠)去除废水中的砷,生成物为难溶性的三硫化二砷,该反 应的离子方程式为_____。
(2)电镀厂的废水中含有的 CN-有剧毒,需要处理加以排放。处理含 CN-废水的方法之一是在 微生物的作用下,CN-被氧气氧化成 HCO3- ,同时生成 NH3,该反应的离子方程式为_____。
(3)电渗析法处理厨房垃极发酵液,同时得到乳酸的原理如图所示(图中“HA”表示乳酸分子, A―表示乳酸根离子):
①阳极的电极反应式为_____
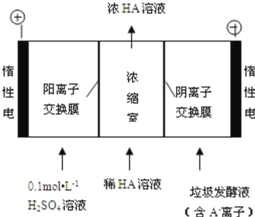
②电解过程中,采取一定的措施可控制阳极室的 pH 约为 6~8,此时进入浓缩室的 OH-可忽略不 计。400 mL 10 g·L-1 乳酸溶液通电一段时间后, 浓度上升为 145 g/L(溶液体积变化忽略不计),阴极上产生的 H2 在标准状况下的体积约为_____L (已知:乳酸的摩尔质量为 90 g/mol)。
【答案】第四周期ⅤA族 2H3AsO3+3S2-+6H+=As2S3↓+6H2O 4H2O+2CN-+O2=2HCO3-+2NH3 4OH--4e-═2H2O+O2↑或2H2O-4e-═O2↑+4H+ 6.72
【解析】
(1)①由砷是氮的同族元素,比氮原子多2个电子层可知,砷原子最外层有5个电子,4个电子层;
②根据题给信息可知,酸性条件下,硫化钠与和废水中的砷酸反应,生成难溶性的三硫化二砷和水;
(2)由题意可知,在微生物的作用下,CN-被氧气氧化成HCO3-,同时生成NH3,反应中碳元素化合价升高被氧化,氮元素化合价没有变化;
(3)①水电离出的氢氧根离子在阳极上失电子发生氧化反应生成氧气,破坏水的电离平衡,使溶液呈酸性;
②由电极反应式和生成HA的化学方程式可得:2HA—2 H+—H2,由此计算可得。
(1)①由砷是氮的同族元素,比氮原子多2个电子层可知,砷原子最外层有5个电子,4个电子层,则砷位于元素周期表第四周期ⅤA族,故答案为:第四周期ⅤA族;
②根据题给信息可知,酸性条件下,硫化钠与和废水中的砷酸反应,生成难溶性的三硫化二砷和水,反应中硫化钠做还原剂,砷酸做氧化剂,反应的离子方程式为2H3AsO3+3S2-+6H+=As2S3↓+6H2O,故答案为:2H3AsO3+3S2-+6H+=As2S3↓+6H2O;
(2)由题意可知,在微生物的作用下,CN-被氧气氧化成HCO3-,同时生成NH3,反应中碳元素化合价升高被氧化,氮元素化合价没有变化,则反应的离子方程式为4H2O+2CN-+O2=2HCO3-+2NH3,故答案为:4H2O+2CN-+O2=2HCO3-+2NH3;
(3)①水电离出的氢氧根离子在阳极上失电子发生氧化反应生成氧气,破坏水的电离平衡,使溶液呈酸性,溶液中氢离子浓度增大,电极反应式为4OH-或2H2O-4e-═O2↑+4H+,故答案为:4OH--4e-═2H2O+O2↑或2H2O-4e-═O2↑+4H+;
②由电极反应式和生成HA的化学方程式可得:2HA—2 H+—H2,电解过程中生成HA的质量为(145 g/L×0.4L—10 g/L×0.4L)=54g,则由HA和氢气的关系式可知阴极上产生的 H2 在标准状况下的体积约为![]() ×
×![]() ×22.4L/mol=6.72L,故答案为:6.72。
×22.4L/mol=6.72L,故答案为:6.72。

【题目】Ⅰ.某小组以CoCl2·6H2O、过氧化氢、液氨、氯化铵固体为原料,在活性炭催化下,合成了橙黄色晶体X。为确定其组成,进行如下实验:
①氨的测定:精确称取wgX,加适量水溶解,注入圆底烧瓶中,然后逐滴加入足量10%NaOH溶液,通入水蒸气,将样品溶液中的氨全部蒸出,用V1mL c1mol·L-1的盐酸溶液吸收。蒸氨结束后取下接收瓶,用c2mol·L-1 NaOH标准溶液滴定过剩的HCl,到终点时消耗V2mLNaOH溶液。
②氯的测定:准确称取样品X配成溶液后用AgNO3标准溶液滴定,K2CrO4溶液为指示剂,至出现砖红色沉淀不在消失为终点(Ag2CrO4为砖红色)。
回答下列问题:
(1)用NaOH标准溶液滴定过剩的HCl时,应使用___式滴定管,该滴定实验可使用的指示剂为___,达到滴定终点的现象为___。
(2)样品中氨的质量分数表达式为___。
(3)滴定终点时,若溶液中c(Ag+)=2.0×10-5mol·L-1 ,c(CrO42-)为___mol·L-1。(已知:Ksp(Ag2CrO4)=1.12×10-12)。
Ⅱ.已知
化合物 | Zn(OH)2 | Fe(OH)2 | Fe(OH)3 |
Ksp近似值 | 10-17 | 10-17 | 10-39 |
(4)则用废电池的锌皮制作七水合硫酸锌,需去除少量杂质铁,其方法是:加入稀硫酸和双氧水,溶解,铁变为___加入___调节pH为___(保留两位有效数字),铁刚好完全沉淀(离子浓度小于1×10-5mol/L时,即可认为该离子沉淀完全)。
【题目】下列实验方案中,可以达到实验目的的是( )
选项 | 实验目的 | 实验方案 |
A | 检验亚硫酸钠是否变质 | 先将亚硫酸钠样品溶于水配成溶液,然后加入足量稀盐酸酸化,再加入 |
B | 除去苯中混有的苯酚 | 加入适量的溴水,充分反应后过滤,弃去沉淀 |
C | 除去NaCl晶体中混有 | 先将晶体溶于水配成溶液,然后蒸发结晶并趁热过滤弃去滤液 |
D | 检验 | 将 |
A.AB.BC.CD.D
【题目】下列实验操作、现象和结论均正确的是
选项 | 操作 | 现象 | 结论 |
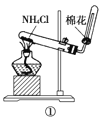
A | ①将湿润的红色石蕊试纸靠近试管口
| 试纸不变色 |
|
B | ②中振荡后静置 | 下层液体颜色变浅 |
|
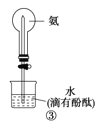
C | ③旋开活塞 | 观察到红色喷泉 |
|
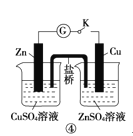
D | ④闭合开关K,形成原电池 | Zn极上有红色固体析出 | 锌的金属性比铜强 |
A.AB.BC.CD.D