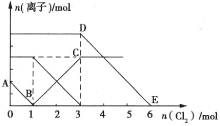
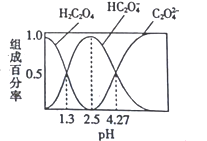
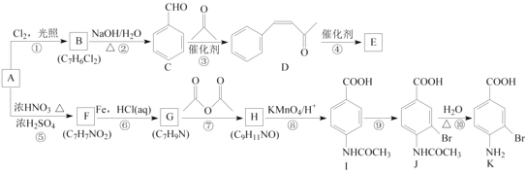
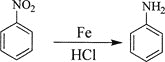
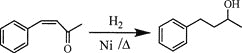
��Ŀ����
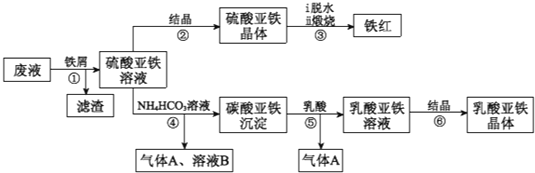
����Ŀ��ij�����Թ�ҵ�����Ѱ۲����ķ�Һ�����ۺ����ã���Һ�к��д���FeSO4��H2SO4������Fe2��SO4��3��TiOSO4��������������������Ͳ�Ѫ�����������������������������£�
��֪����TiOSO4������ˮ����ˮ�п��Ե���ΪTiO2+��SO42-��
��TiOSO4ˮ��ķ�ӦΪ��TiOSO4+��x+1��H2O��TiO2xH2O��+H2SO4��
��ش�
��1��������������������Ҫ�ɷ�Ϊ_____________��
��2�����������������ڿ�������������������������÷�Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ_____________��
��3������������Ʒ�Ӧ�¶ȵ���35������Ŀ����_____________��
��4�������������ӷ���ʽ��_____________��
��5����֪��FeCO3��S��![]() Fe2+��aq��+CO32-��aq��������ƽ���ƶ�ԭ�����Ͳ�������������������ԭ��_____________��
Fe2+��aq��+CO32-��aq��������ƽ���ƶ�ԭ�����Ͳ�������������������ԭ��_____________��
��6����ҺB�������ڵ��������NH4��2S2O8������������������ʱ���ö��Ե缫�����������ĵ缫��Ӧ�ɱ�ʾΪ_____________��
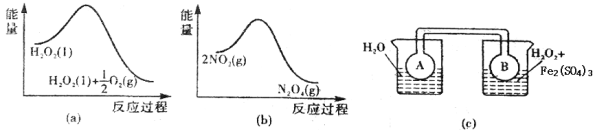
��7��Fe3+��H2O2�ķֽ���д����ã�����ͼ2��a������b���е���Ϣ����ͼ2��c��װ������ͨ��A��Bƿ���ѳ���NO2����������ʵ�飮�ɹ۲쵽Bƿ��������ɫ��Aƿ�е����ԭ����_____________��
���𰸡�
��1��TiO2xH2O��Fe��
��2��1:4��
��3����ֹNH4HCO3�ֽ��������Fe3+ˮ����
��4��Fe2++2HCO3-=FeCO3��+H2O+CO2����
��5��FeCO3��s��![]() Fe2+��aq��+CO32-��aq����CO32-�����ᷴӦŨ�Ƚ��ͣ�ƽ�������ƶ���ʹ̼�������ܽ�õ�����������Һ��
Fe2+��aq��+CO32-��aq����CO32-�����ᷴӦŨ�Ƚ��ͣ�ƽ�������ƶ���ʹ̼�������ܽ�õ�����������Һ��
��6��2SO42��+2e-=S2O82-
��7��H2O2�ֽ������ʹƽ��2NO2![]() N2O4������NO2�����ƶ���
N2O4������NO2�����ƶ���
��������
�����������Һ�к��д���FeSO4��H2SO4������Fe2��SO4��3��TiOSO4������м��Fe��H2SO4������Fe2��SO4��3��Ӧ����FeSO4��TiOSO4ˮ������TiO2xH2O�����ˣ�����ΪTiO2xH2O��Fe����ҺΪFeSO4��FeSO4��Һͨ������Ũ������ȴ�ᾧ������ϴ�ӵõ������������壬��ˮ�����յõ���������FeSO4��Һ�м�̼�����̼����������������狀Ͷ�����̼��̼�����������������ܽ���������������Һ�Ͷ�����̼������������Һͨ������Ũ������ȴ�ᾧ������ϴ�ӵõ������������塣
��1�������̷�����֪��������������������Ҫ�ɷ�ΪTiO2xH2O��Fe���ʴ�Ϊ��TiO2xH2O��Fe��
��2�����������ڿ��������������������������ķ���ʽΪ��4FeSO4+O2![]() 2Fe2O3+4SO3������������������ԭ�����������������������ͻ�ԭ�������ʵ���֮��Ϊ1��4���ʴ�Ϊ��1��4��
2Fe2O3+4SO3������������������ԭ�����������������������ͻ�ԭ�������ʵ���֮��Ϊ1��4���ʴ�Ϊ��1��4��
��3��̼������������ֽ⣬���������Ʒ�Ӧ�¶ȵ���35����Ŀ������ֹNH4HCO3�ֽ⣬�ʴ�Ϊ����ֹNH4HCO3�ֽ⣻
��4��������ͼ��֪������������̼����立�Ӧ��̼������������������Ϊ������̼����ҺBΪ�������Һ����Ӧ���ӷ���ʽΪ��Fe2++2HCO3-=FeCO3��+H2O+CO2�����ʴ�Ϊ��Fe2++2HCO3-=FeCO3��+H2O+CO2����
��5��̼�������ij��������ܽ�ƽ�⣺FeCO3��s��![]() Fe2+��aq��+CO32-��aq�����������ᣬ����CO32-�����ᷴӦŨ�Ƚ��ͣ�ƽ�������ƶ���ʹ̼�������ܽ�õ�����������Һ���ʴ�Ϊ��FeCO3��s��
Fe2+��aq��+CO32-��aq�����������ᣬ����CO32-�����ᷴӦŨ�Ƚ��ͣ�ƽ�������ƶ���ʹ̼�������ܽ�õ�����������Һ���ʴ�Ϊ��FeCO3��s��![]() Fe2+��aq��+CO32-��aq����CO32-�����ᷴӦŨ�Ƚ��ͣ�ƽ�������ƶ���ʹ̼�������ܽ�õ�����������Һ��
Fe2+��aq��+CO32-��aq����CO32-�����ᷴӦŨ�Ƚ��ͣ�ƽ�������ƶ���ʹ̼�������ܽ�õ�����������Һ��
��6�����ʱ����������������Ӧ�������������ʧȥ��������S2O82-���缫��ӦΪ2SO42��+2e-=S2O82-���ʴ�Ϊ��2SO42��+2e-=S2O82-��
��7����ͼa��֪��1mol������������������1molˮ��0.5mol�������������ʹ�������ֽ��Ƿ��ȷ�Ӧ����ͼb��֪��2mol������������������1mol�������������������ʶ�������ת��Ϊ�����������ķ�ӦΪ���ȷ�Ӧ������ͼc�У��Ҳ��ձ����¶ȸ�����࣬�����¶�ʹ2NO2������ɫ��![]() N2O4����ɫ����H��0�����淴Ӧ�����ƶ�����������NO2�ƶ�����Bƿ��ɫ����ʴ�Ϊ��H2O2�ֽ���ȣ�ʹƽ��2NO2
N2O4����ɫ����H��0�����淴Ӧ�����ƶ�����������NO2�ƶ�����Bƿ��ɫ����ʴ�Ϊ��H2O2�ֽ���ȣ�ʹƽ��2NO2![]() N2O4������NO2�����ƶ���
N2O4������NO2�����ƶ���