��Ŀ����
CO2��CO�ǹ�ҵ�ŷŵĶԻ�������Ӱ��ķ�����
��1����CO2��NH3Ϊԭ�Ϻϳɻ������ص���Ҫ��Ӧ���£�
��2NH3(g)��CO2(g)��NH2CO2NH4(s)����H����159.47 kJ��mol��1
��NH2CO2NH4(s)��CO(NH2)2(s)��H2O(g)����H��a kJ��mol��1
��2NH3(g)��CO2(g)��CO(NH2)2(s)��H2O(g)����H����86.98 kJ��mol��1
��aΪ ��
��2����ѧ��������ù�ҵ�����е�CO2��ȡ�״���CO2+3H2CH3OH+H2O���Ƶõ�CH3OH������ȼ�ϵ�ص�ȼ�ϡ�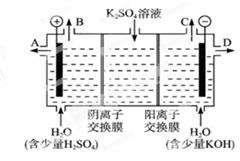
����KOH�����У������ĵ缫��ӦʽΪ_________________________________��
�������ʵ�KOH�����õ��K2SO4��Һ�ķ����Ƶá���KOH��_______���ڵõ�,�����ĵ缫��Ӧʽ�ǣ�_____________________________________��
��3������CO��H2��Ӧ�ɺϳ�CH3OCH3��
��֪��3H2(g) + 3CO(g)  CH3OCH3(g) + CO2(g)����H��-247kJ/mol
CH3OCH3(g) + CO2(g)����H��-247kJ/mol
��һ�������µ��ܱ������У��÷�Ӧ�ﵽƽ�⣬Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ�� .
| A�����¸�ѹ�� | B����������� | C�����������뺤���� | D������CO��Ũ�ȣ�E������������� |
 CH3OCH3(g) + H2O(g)����ij�¶��£���1L�ܱ������м���CH3OH ����Ӧ��10����ʱ�ﵽƽ�⣬��ʱ��ø���ֵ�Ũ�����£�
CH3OCH3(g) + H2O(g)����ij�¶��£���1L�ܱ������м���CH3OH ����Ӧ��10����ʱ�ﵽƽ�⣬��ʱ��ø���ֵ�Ũ�����£�| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ��/��mol��L��1�� | 0.01 | 0.2 | 0.2 |
��0-10 min�ڷ�Ӧ����v(CH3OH) �� ��
�ڸ��¶��µ�ƽ�ⳣ��Ϊ ��
����ƽ��������������ټ���0.01mol CH3OH��0.2mol CH3OCH3����ʱ�����淴Ӧ���ʵĴ�С��
v�� v�� ���>������<������)��
��1��+72.49kJ��mol��1 ��2�֣�
��2����CH3OH -6e-+8OH-=CO32-+6H2O��2�֣�
��D ��2�֣�4OH- + 4e- = 2H2O + O2�� ��2�֣�
��3��AE ��2�֣�
��4����0.04 mol��L-1��min-1��2�֣��� 400��2�֣� �� �� ��2��
���������������1�������Ȼ�ѧ����ʽ��˹���ɢ�+��=�ۣ��õ�-159.47KJ/mol+a=-86.98KJ/mol��a=+72.49KJ/mol��
��2���ټ״���ȼ���Ƴɵ�ȼ�ϵ�����ɵ�CO2������Һ��������K2CO3���ܷ�ӦʽΪ��2CH3OH+4KOH+3O2=2K2CO3+6H2O,���Լ״��ڸ���ʧȥ���ӣ������ĵ缫��ӦʽΪ��2CH3OH-12e- +16OH- =2CO32- +12H2O��������Ϊ��3O2+12e-+6H2O=12OH-
�ڵ��K2SO4��Һʵ�ʾ��ǵ��ˮ������ˮ�����OH-ʧȥ���ӱ�������������������H+��SO42-ͨ�������ӽ���Ĥ������������A�ڳ��õ����ᣬ�����缫��ӦʽΪ��4OH- + 4e- = 2H2O + O2����������ˮ�����H+�õ����ӱ���ԭ�����Ի��д���OH-���ɣ�K+ͨ�������ӽ���Ĥ������������D�ڳ��õ�KOH��������ӦʽΪ:4H++ 4e- =2H2����
��3���ɷ�Ӧ��֪����ӦΪ���ȡ�ǰ������ϵ�����ٵķ�Ӧ����������ѹǿ�������¶�����������Ӧ���CO��ת���ʣ�����A���У��������ֻ�ܼӿ췴Ӧ���ʣ����ܸı�ƽ�⣬B�����У����������뺤�����ı���μӷ�Ӧ�����Ũ�ȣ�����ı�ƽ�⣬���C������COת���ʣ�����CO��Ũ����ʹƽ�������ƶ�������CO��ת���ʷ������ͣ�E����������������ѣ�����ʹ��Ӧ�����ƶ������������CO��ת���ʣ�����E���У���ѡAE��
��4�����ݱ��������У�2CH3OH(g)  CH3OCH3(g) + H2O(g)
CH3OCH3(g) + H2O(g)
��ʼŨ�ȣ�mol/L���� 0.41 0 0
ת��Ũ�ȣ�mol/L���� 0.4 0.2 0.2
ƽ��Ũ�ȣ�mol/L���� 0.01 0.2 0.2
���Ԣ�0-10 min�ڷ�Ӧ����v(CH3OH) ��0.4 mol/L��10min=0.04 mol��L-1��min-1
�ڸ��¶��µ�ƽ�ⳣ��K=0.2��0.2��0.012 =400
����Ϊ��ƽ��ʱ����������0.01mol CH3OH��0.2mol CH3OCH3 ��0.2mol H2O�������º����������������ټ���ͬ����0.01mol CH3OH��0.2mol CH3OCH3��0.2mol H2O����ﵽ��Чƽ�⣬ƽ�ⲻ�ƶ�������ֻ����0.01mol CH3OH��0.2mol CH3OCH3 ���ܴﵽ��Чƽ�⣬ƽ��������Ӧ�����ƶ������Դ�ʱv��>v�� ��
���㣺���⿼����ǻ�ѧ��Ӧ���������绯ѧ��������ѧ��Ӧ������ƽ���֪ʶ���ۺϡ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д����ǵ����Ϻ�����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���á���ش��������⣺
��1����ͼ��1 mol NO2��1mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ ��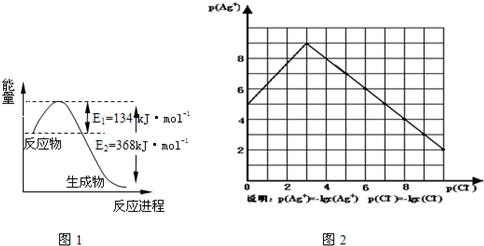
��2����0.5L���ܱ������У�һ�����ĵ����������������»�ѧ��Ӧ��N2(g)+3H2(g)  2NH3(g)��H<0�仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���ұ���������������⣻
2NH3(g)��H<0�仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���ұ���������������⣻
| t/�� | 200 | 300 | 400 |
| K | K1 | K2 | 0.5 |
���ԱȽ�K1��K2�Ĵ�С��K1____K2����д��>������=����<����
�����и�������Ϊ�жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��������____һ���������ĸ��
a��������N2��H2��NH3��Ũ��֮��Ϊ1��3��2
b��v��N2����=3v��H2����
c��������ѹǿ���ֲ���
d�����������ܶȱ��ֲ���
��400��ʱ����Ӧ2NH3(g)
 N2(g)+3H2(g)�Ļ�ѧƽ�ⳣ����ֵΪ �������NH3��N2��H2�����ʵ����ֱ�Ϊ3mol��2mol��1molʱ����÷�Ӧ��v��N2���� __ v��N2��������д��>������=����<��=��
N2(g)+3H2(g)�Ļ�ѧƽ�ⳣ����ֵΪ �������NH3��N2��H2�����ʵ����ֱ�Ϊ3mol��2mol��1molʱ����÷�Ӧ��v��N2���� __ v��N2��������д��>������=����<��=�� ����һ�ֵ����Ϻ����ḻ��Ԫ�أ������仯������о���������������������Ҫ���塣
��1����ͼ��1 mol NO2��1 mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ��д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ ��
��2����֪��N2(g)+ O2(g)��2 NO(g) ��H����180 kJ ? mol-1
2NO(g)+2 CO(g)��N2(g) + 2 CO2(g) ��H����746 kJ ? mol-1
��ӦCO(g) + O2(g)��CO2(g)�� ��H�� kJ ? mol-1��
O2(g)��CO2(g)�� ��H�� kJ ? mol-1��
��3����һ�̶��ݻ�Ϊ2L���ܱ������ڼ���0.2 mol��N2��0.6 mol��H2����һ�������·������·�Ӧ�� N2(g)��3H2(g) 2NH3(g) ��H ��0�� ����5����ʱ�ﵽƽ�⣬��ʱ���NH3�����ʵ���Ϊ0.2 mol��ƽ��ʱH2��ת����Ϊ ��
2NH3(g) ��H ��0�� ����5����ʱ�ﵽƽ�⣬��ʱ���NH3�����ʵ���Ϊ0.2 mol��ƽ��ʱH2��ת����Ϊ ��
��4���ڹ̶�������ܱ������У�1.0��103 kPaʱ��������Ӧ N2(g)+3H2(g) 2NH3(g) ��H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
2NH3(g) ��H��0����ƽ�ⳣ��K���¶�T�Ĺ�ϵ���±���
| T/K | 298 | 398 | 498 |
| ƽ�ⳣ��K | 51 | K1 | K2 |
�� K1 K2�����������������������
�����и�����˵�������ϳɰ���Ӧһ���ﵽƽ��״̬���� ������ĸ����
a��������N2��H2��NH3��Ũ��֮��Ϊ1:3:2
b��NH3��Ũ�ȱ��ֲ���
c��������ѹǿ���ֲ���
d�����������ܶȱ��ֲ���
��ѧ��Ӧ��һ�������������ı仯����ѧ����ʵ�����������е�Ӧ�ý�Խ��Խ�㷺��
��1����������������ȡ��ҵԭ�����ᡣ��֪��
��CH3COOH(l)+2O2(g)=2CO2(g)+2H2O(l) ��H1 = ��870.3kJ��mo1
��C(s)+O2(g)=CO2(g) ��H 2= ��393.5kJ��mo1
��H2(g) + 1/2O2(g)=H2O(l) ��H 3= ��285.8kJ��mo1
������������Ϣ����������Ӧ�ķ�Ӧ�ȣ�
2C(s)+2H2(g)+O2(g)=CH3COOH(l) ��H= ��
��2����ѧ�ҿ����ʱ���ر����жϻ�ѧ��Ӧ���еķ������ж������Է����еĻ�ѧ��Ӧ�����������ȷ�Ӧ�ҷ�Ӧ��������Ϊ ������ĸ����
| A��2Na+2H2O=2NaOH+H2�� | B��3Fe+2O2=Fe3O4 |
| C��(NH4)2CO3=NH4HCO3+NH3�� | D��NaOH+HCl=NaCl+H2O |

����ָ����װ�û�ȱ�ٵ������� ��
�����ձ�����������ĭ���ϵ������� ��
���������ݵ�����������ʵ���Ũ�ȵ�NaOH��Һ�зֱ����ϡ���ᡢŨ���ᡢϡ������ǡ����ȫ��Ӧ���������������зų��������ֱ��ΪQl��Q2��Q3�������ߵĴ�С��ϵ�� ���ɴ�С����
 [Cu��NH3��3]Ac?CO��aq������H��0��
[Cu��NH3��3]Ac?CO��aq������H��0��
 CH3OH(g)��ͼ1��ʾ��Ӧ�е������仯��ͼ2��ʾһ���¶��£������Ϊ1L���ܱ������м���2mol H2��һ������CO��CO��CH3OH(g)��Ũ����ʱ��仯��
CH3OH(g)��ͼ1��ʾ��Ӧ�е������仯��ͼ2��ʾһ���¶��£������Ϊ1L���ܱ������м���2mol H2��һ������CO��CO��CH3OH(g)��Ũ����ʱ��仯��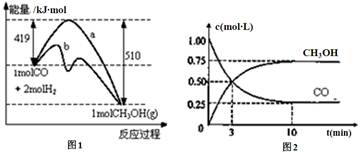
 CH3OH(g)�Ļ�ѧƽ�ⳣ��Ϊ ���ﵽƽ��������������������䣬���������ѹ��Ϊ0.5L����ƽ�� �ƶ� (�������������)��
CH3OH(g)�Ļ�ѧƽ�ⳣ��Ϊ ���ﵽƽ��������������������䣬���������ѹ��Ϊ0.5L����ƽ�� �ƶ� (�������������)��
 CH3OH(g)
CH3OH(g)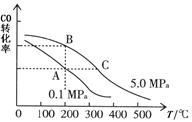
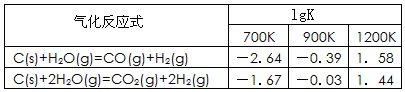
 CO2(g)+H2(g)���÷�Ӧ�ġ�H________0��ѡ�����������������������������900Kʱ���÷�Ӧƽ�ⳣ���Ķ���ֵ��lgK��=_____________.
CO2(g)+H2(g)���÷�Ӧ�ġ�H________0��ѡ�����������������������������900Kʱ���÷�Ӧƽ�ⳣ���Ķ���ֵ��lgK��=_____________.